| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 148721 | Chemical Engineering Journal | 2013 | 8 Pages |
•Synthesis of hierarchical Bi24O31Br10 nanoflakes by a microwave heating route.•The formation mechanism of Bi24O31Br10 nanoflakes is proposed.•The visible-light photocatalyst is Bi24O31Br10 nanoflakes.•Bi24O31Br10 nanoflakes have photocatalytic ability to tetracycline hydrochloride.
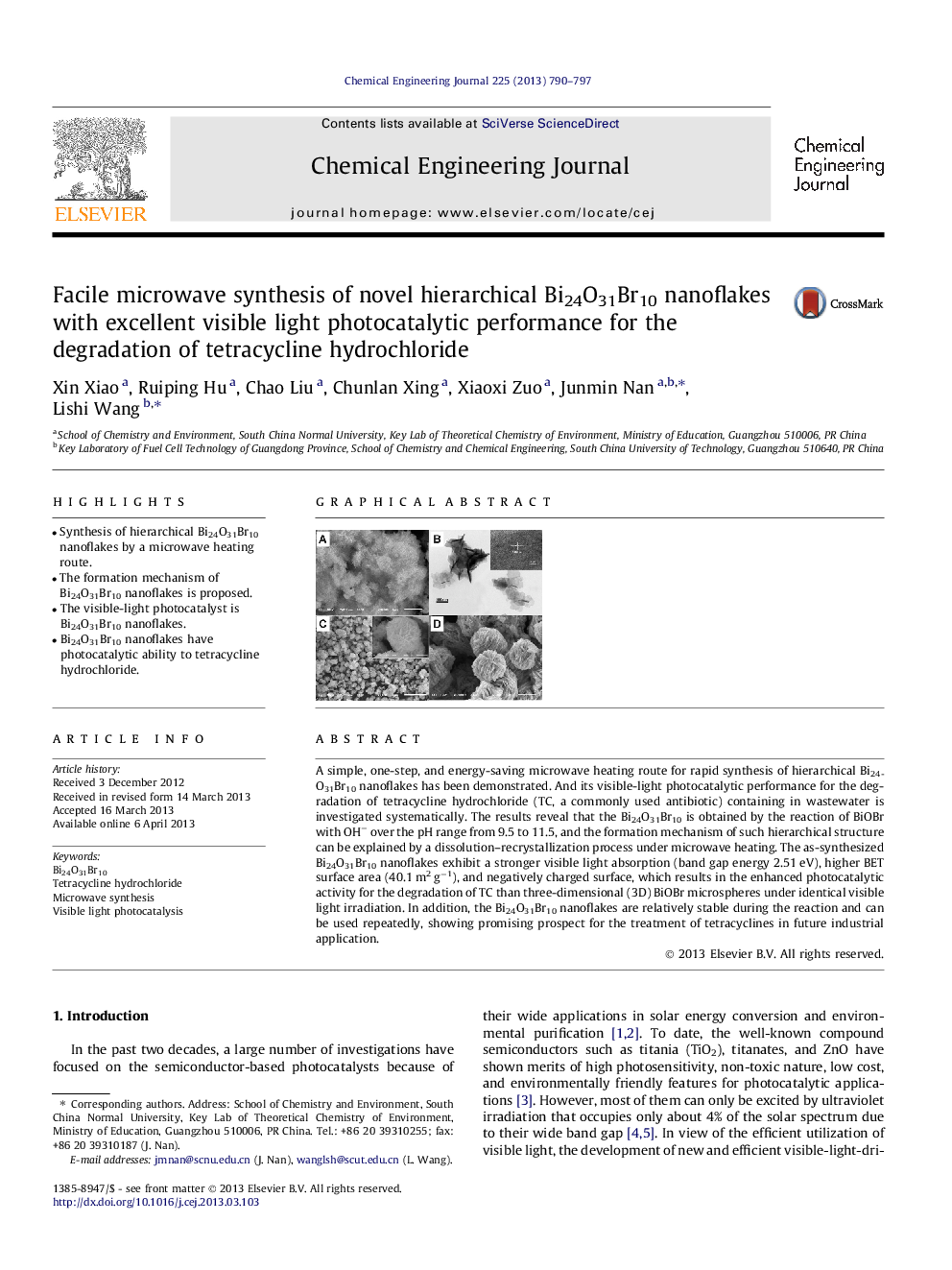
A simple, one-step, and energy-saving microwave heating route for rapid synthesis of hierarchical Bi24O31Br10 nanoflakes has been demonstrated. And its visible-light photocatalytic performance for the degradation of tetracycline hydrochloride (TC, a commonly used antibiotic) containing in wastewater is investigated systematically. The results reveal that the Bi24O31Br10 is obtained by the reaction of BiOBr with OH− over the pH range from 9.5 to 11.5, and the formation mechanism of such hierarchical structure can be explained by a dissolution–recrystallization process under microwave heating. The as-synthesized Bi24O31Br10 nanoflakes exhibit a stronger visible light absorption (band gap energy 2.51 eV), higher BET surface area (40.1 m2 g−1), and negatively charged surface, which results in the enhanced photocatalytic activity for the degradation of TC than three-dimensional (3D) BiOBr microspheres under identical visible light irradiation. In addition, the Bi24O31Br10 nanoflakes are relatively stable during the reaction and can be used repeatedly, showing promising prospect for the treatment of tetracyclines in future industrial application.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide