| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 149016 | Chemical Engineering Journal | 2013 | 12 Pages |
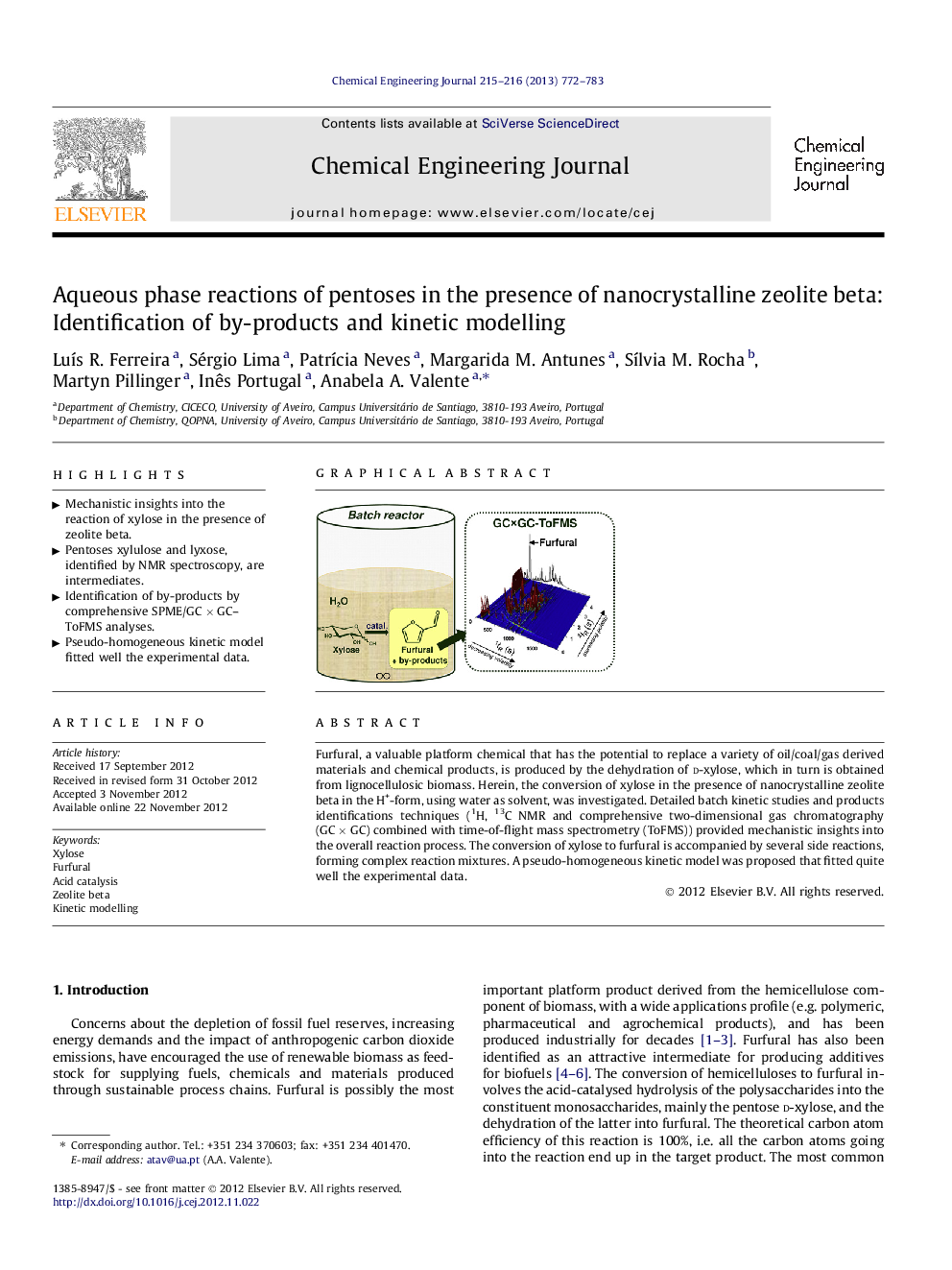
Furfural, a valuable platform chemical that has the potential to replace a variety of oil/coal/gas derived materials and chemical products, is produced by the dehydration of d-xylose, which in turn is obtained from lignocellulosic biomass. Herein, the conversion of xylose in the presence of nanocrystalline zeolite beta in the H+-form, using water as solvent, was investigated. Detailed batch kinetic studies and products identifications techniques (1H, 13C NMR and comprehensive two-dimensional gas chromatography (GC × GC) combined with time-of-flight mass spectrometry (ToFMS)) provided mechanistic insights into the overall reaction process. The conversion of xylose to furfural is accompanied by several side reactions, forming complex reaction mixtures. A pseudo-homogeneous kinetic model was proposed that fitted quite well the experimental data.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights• Mechanistic insights into the reaction of xylose in the presence of zeolite beta. • Pentoses xylulose and lyxose, identified by NMR spectroscopy, are intermediates. • Identification of by-products by comprehensive SPME/GC × GC–ToFMS analyses. • Pseudo-homogeneous kinetic model fitted well the experimental data.