| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 149037 | Chemical Engineering Journal | 2013 | 10 Pages |
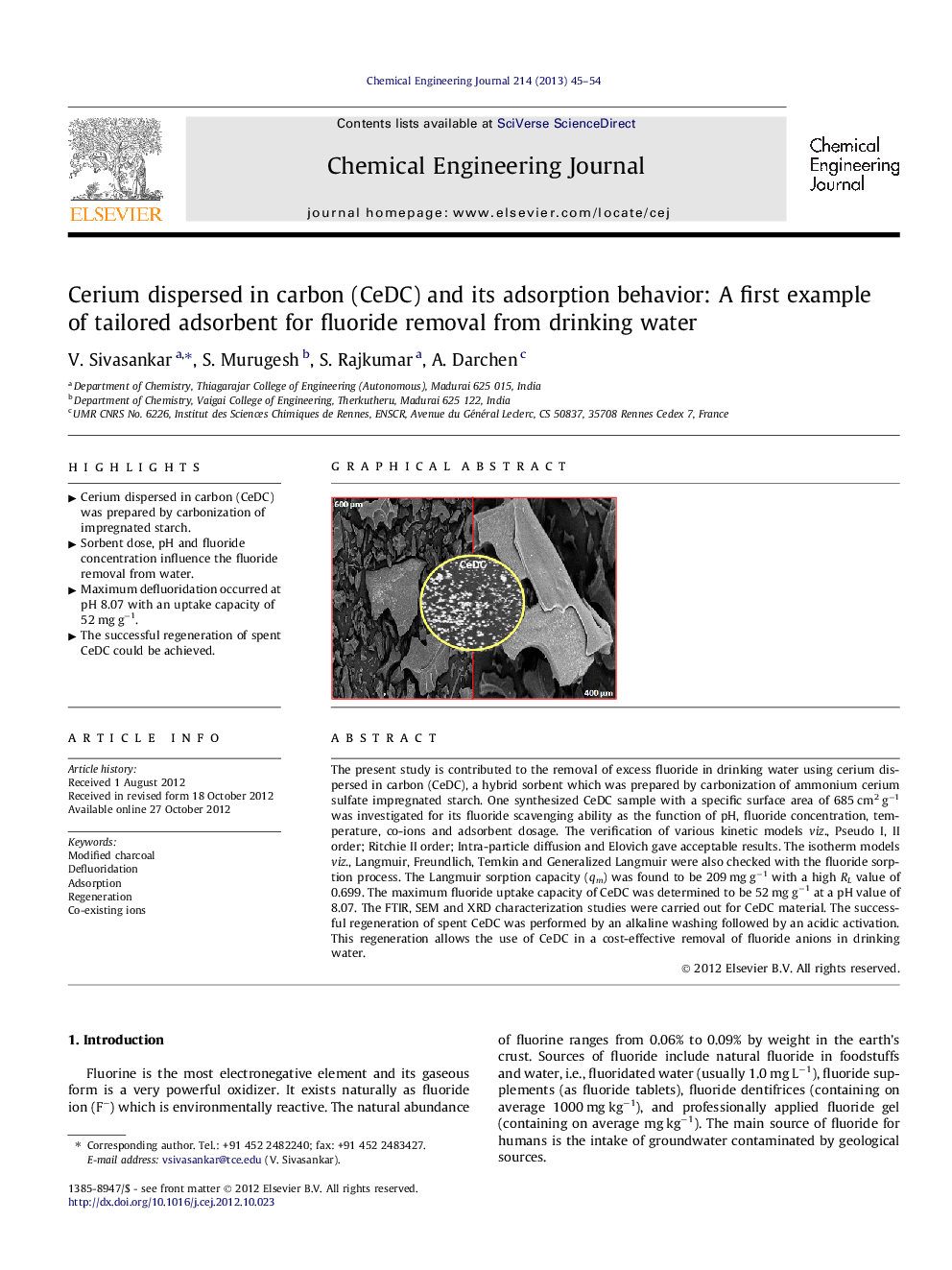
The present study is contributed to the removal of excess fluoride in drinking water using cerium dispersed in carbon (CeDC), a hybrid sorbent which was prepared by carbonization of ammonium cerium sulfate impregnated starch. One synthesized CeDC sample with a specific surface area of 685 cm2 g−1 was investigated for its fluoride scavenging ability as the function of pH, fluoride concentration, temperature, co-ions and adsorbent dosage. The verification of various kinetic models viz., Pseudo I, II order; Ritchie II order; Intra-particle diffusion and Elovich gave acceptable results. The isotherm models viz., Langmuir, Freundlich, Temkin and Generalized Langmuir were also checked with the fluoride sorption process. The Langmuir sorption capacity (qm) was found to be 209 mg g−1 with a high RL value of 0.699. The maximum fluoride uptake capacity of CeDC was determined to be 52 mg g−1 at a pH value of 8.07. The FTIR, SEM and XRD characterization studies were carried out for CeDC material. The successful regeneration of spent CeDC was performed by an alkaline washing followed by an acidic activation. This regeneration allows the use of CeDC in a cost-effective removal of fluoride anions in drinking water.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Cerium dispersed in carbon (CeDC) was prepared by carbonization of impregnated starch. ► Sorbent dose, pH and fluoride concentration influence the fluoride removal from water. ► Maximum defluoridation occurred at pH 8.07 with an uptake capacity of 52 mg g−1. ► The successful regeneration of spent CeDC could be achieved.