| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 149101 | Chemical Engineering Journal | 2012 | 10 Pages |
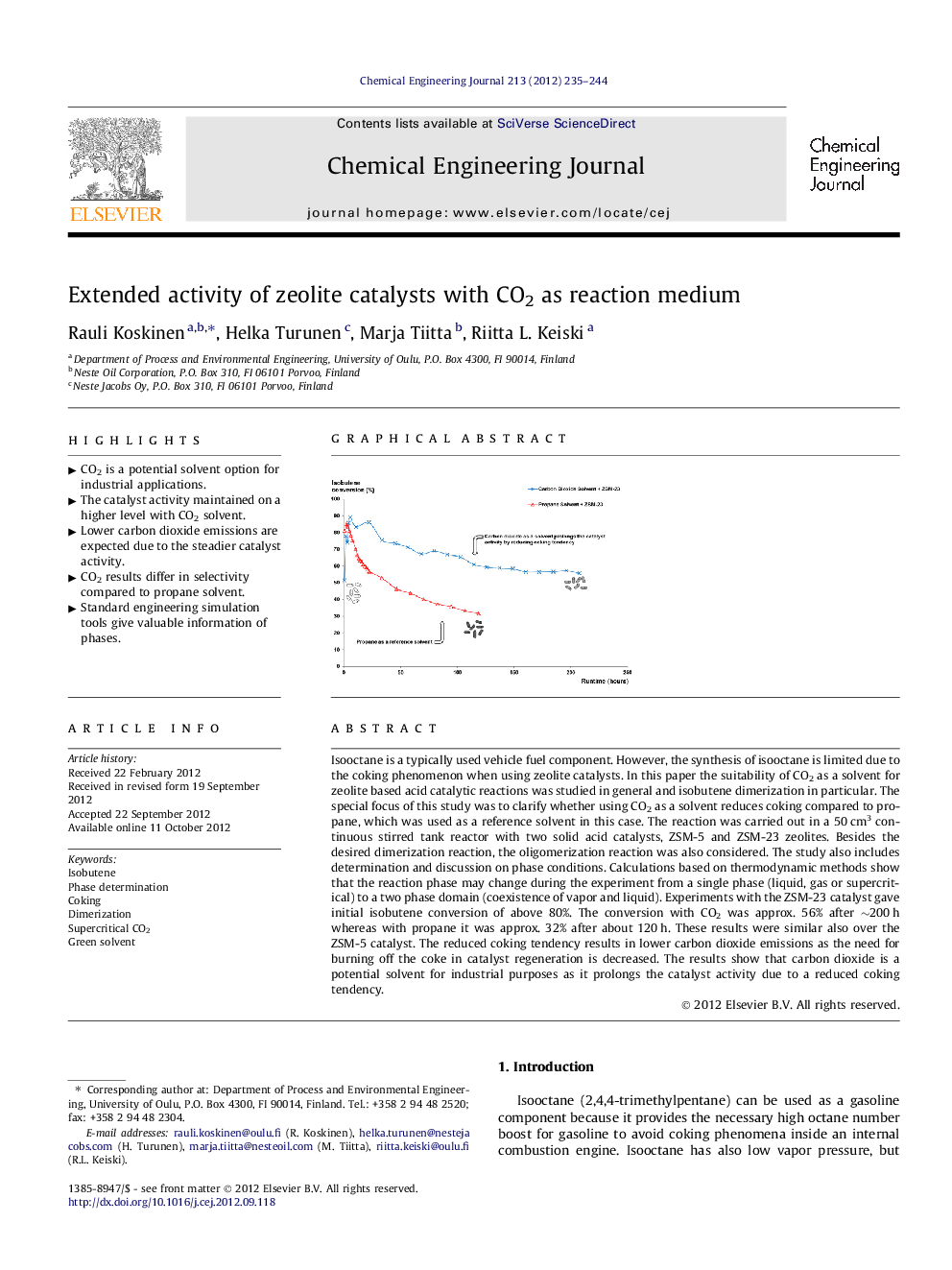
Isooctane is a typically used vehicle fuel component. However, the synthesis of isooctane is limited due to the coking phenomenon when using zeolite catalysts. In this paper the suitability of CO2 as a solvent for zeolite based acid catalytic reactions was studied in general and isobutene dimerization in particular. The special focus of this study was to clarify whether using CO2 as a solvent reduces coking compared to propane, which was used as a reference solvent in this case. The reaction was carried out in a 50 cm3 continuous stirred tank reactor with two solid acid catalysts, ZSM-5 and ZSM-23 zeolites. Besides the desired dimerization reaction, the oligomerization reaction was also considered. The study also includes determination and discussion on phase conditions. Calculations based on thermodynamic methods show that the reaction phase may change during the experiment from a single phase (liquid, gas or supercritical) to a two phase domain (coexistence of vapor and liquid). Experiments with the ZSM-23 catalyst gave initial isobutene conversion of above 80%. The conversion with CO2 was approx. 56% after ∼200 h whereas with propane it was approx. 32% after about 120 h. These results were similar also over the ZSM-5 catalyst. The reduced coking tendency results in lower carbon dioxide emissions as the need for burning off the coke in catalyst regeneration is decreased. The results show that carbon dioxide is a potential solvent for industrial purposes as it prolongs the catalyst activity due to a reduced coking tendency.
Graphical abstract.Figure optionsDownload full-size imageDownload as PowerPoint slideHighlights► CO2 is a potential solvent option for industrial applications. ► The catalyst activity maintained on a higher level with CO2 solvent. ► Lower carbon dioxide emissions are expected due to the steadier catalyst activity. ► CO2 results differ in selectivity compared to propane solvent. ► Standard engineering simulation tools give valuable information of phases.