| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 149230 | Chemical Engineering Journal | 2012 | 8 Pages |
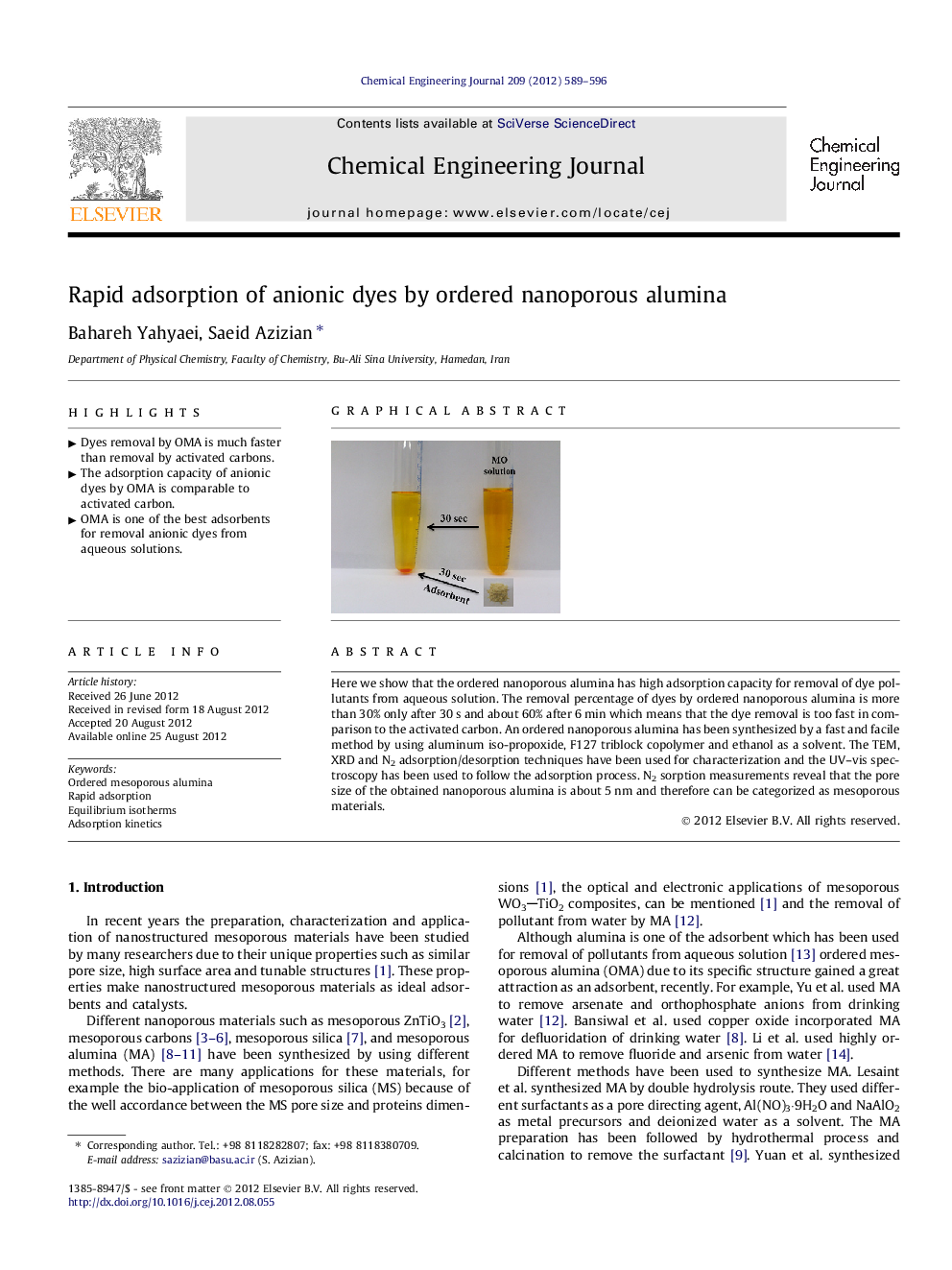
Here we show that the ordered nanoporous alumina has high adsorption capacity for removal of dye pollutants from aqueous solution. The removal percentage of dyes by ordered nanoporous alumina is more than 30% only after 30 s and about 60% after 6 min which means that the dye removal is too fast in comparison to the activated carbon. An ordered nanoporous alumina has been synthesized by a fast and facile method by using aluminum iso-propoxide, F127 triblock copolymer and ethanol as a solvent. The TEM, XRD and N2 adsorption/desorption techniques have been used for characterization and the UV–vis spectroscopy has been used to follow the adsorption process. N2 sorption measurements reveal that the pore size of the obtained nanoporous alumina is about 5 nm and therefore can be categorized as mesoporous materials.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Dyes removal by OMA is much faster than removal by activated carbons. ► The adsorption capacity of anionic dyes by OMA is comparable to activated carbon. ► OMA is one of the best adsorbents for removal anionic dyes from aqueous solutions.