| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 149254 | Chemical Engineering Journal | 2012 | 6 Pages |
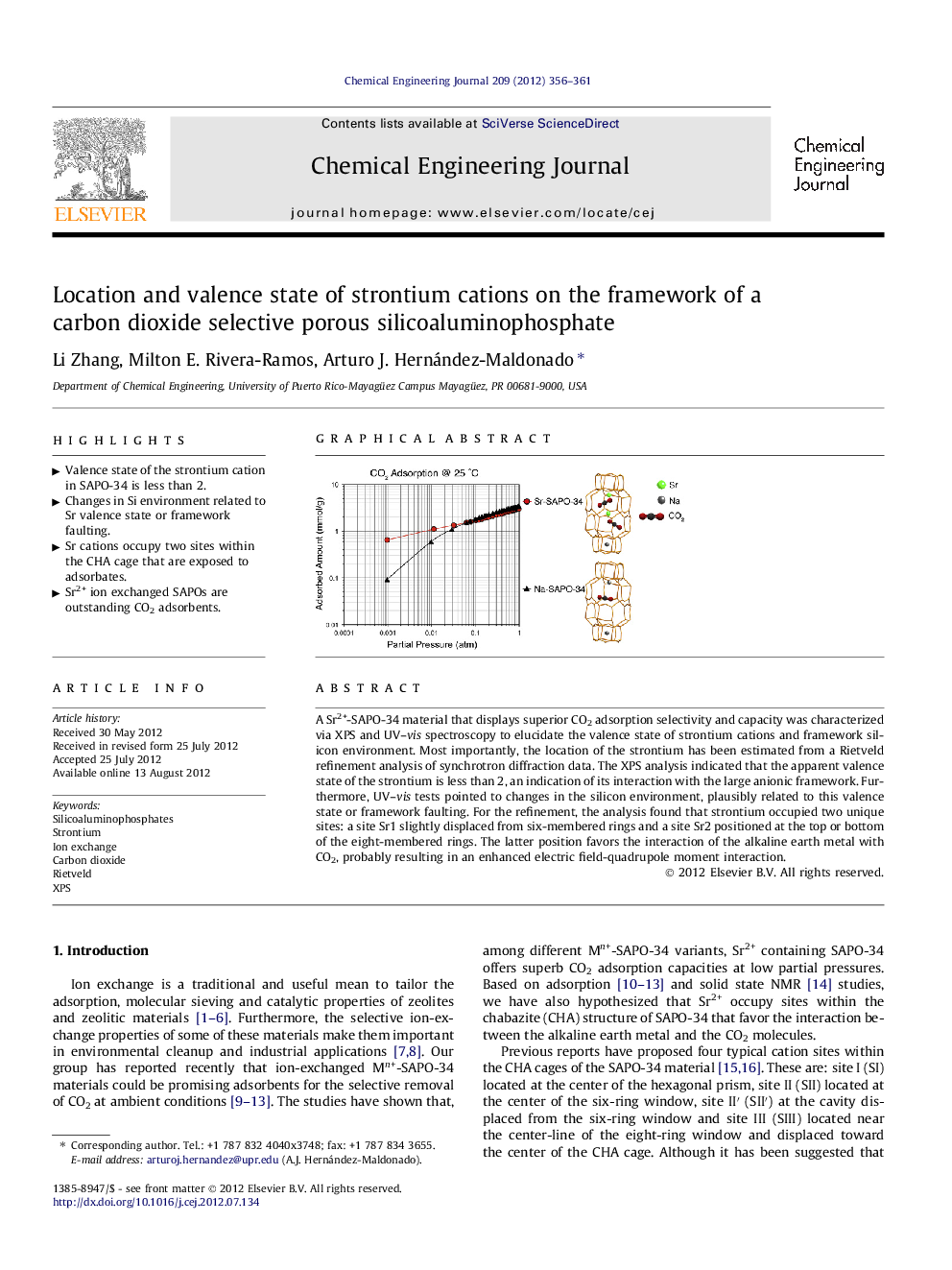
A Sr2+-SAPO-34 material that displays superior CO2 adsorption selectivity and capacity was characterized via XPS and UV–vis spectroscopy to elucidate the valence state of strontium cations and framework silicon environment. Most importantly, the location of the strontium has been estimated from a Rietveld refinement analysis of synchrotron diffraction data. The XPS analysis indicated that the apparent valence state of the strontium is less than 2, an indication of its interaction with the large anionic framework. Furthermore, UV–vis tests pointed to changes in the silicon environment, plausibly related to this valence state or framework faulting. For the refinement, the analysis found that strontium occupied two unique sites: a site Sr1 slightly displaced from six-membered rings and a site Sr2 positioned at the top or bottom of the eight-membered rings. The latter position favors the interaction of the alkaline earth metal with CO2, probably resulting in an enhanced electric field-quadrupole moment interaction.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Valence state of the strontium cation in SAPO-34 is less than 2. ► Changes in Si environment related to Sr valence state or framework faulting. ► Sr cations occupy two sites within the CHA cage that are exposed to adsorbates. ► Sr2+ ion exchanged SAPOs are outstanding CO2 adsorbents.