| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 149332 | Chemical Engineering Journal | 2012 | 7 Pages |
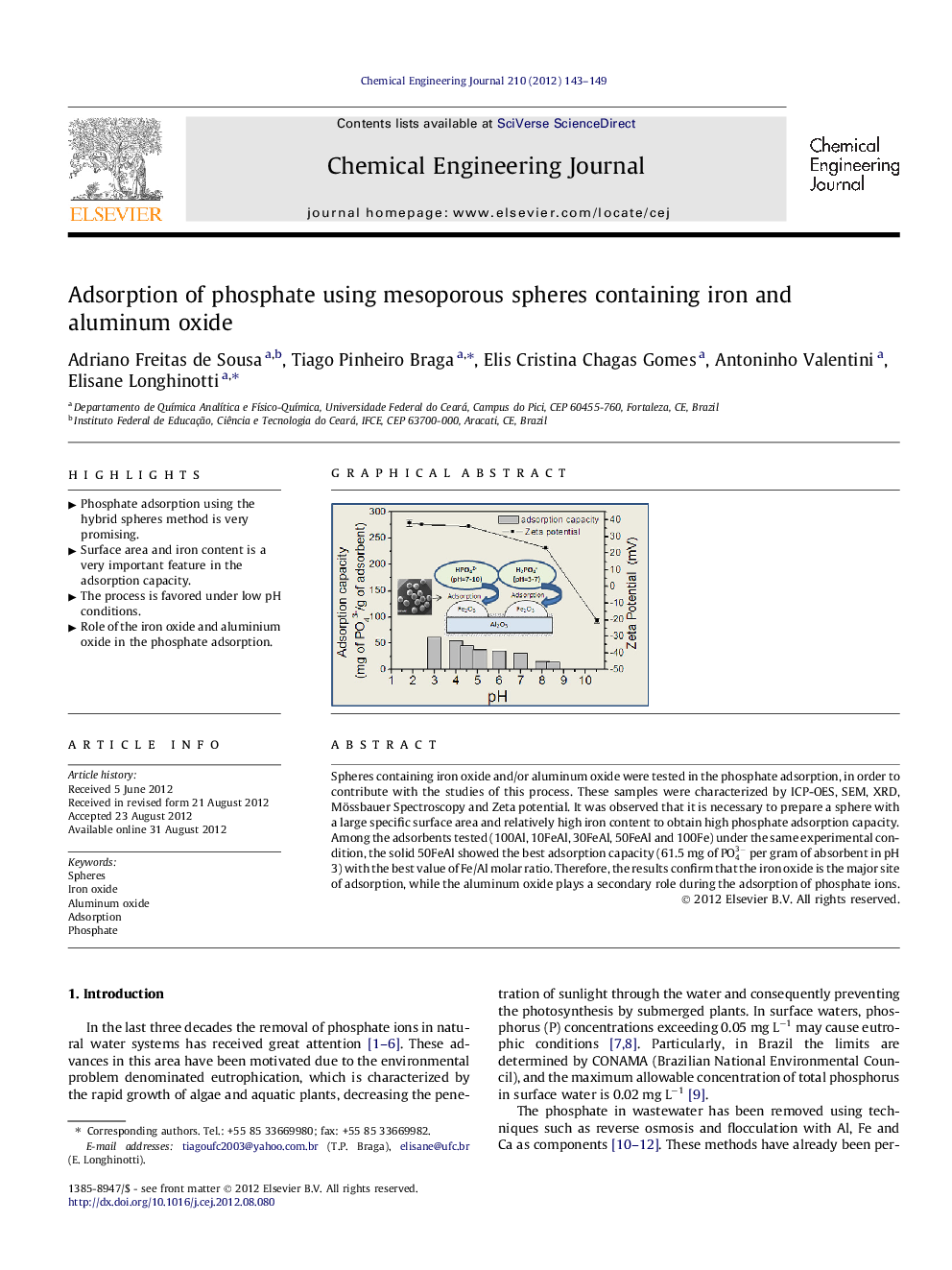
Spheres containing iron oxide and/or aluminum oxide were tested in the phosphate adsorption, in order to contribute with the studies of this process. These samples were characterized by ICP-OES, SEM, XRD, Mössbauer Spectroscopy and Zeta potential. It was observed that it is necessary to prepare a sphere with a large specific surface area and relatively high iron content to obtain high phosphate adsorption capacity. Among the adsorbents tested (100Al, 10FeAl, 30FeAl, 50FeAl and 100Fe) under the same experimental condition, the solid 50FeAl showed the best adsorption capacity (61.5 mg of PO43- per gram of absorbent in pH 3) with the best value of Fe/Al molar ratio. Therefore, the results confirm that the iron oxide is the major site of adsorption, while the aluminum oxide plays a secondary role during the adsorption of phosphate ions.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Phosphate adsorption using the hybrid spheres method is very promising. ► Surface area and iron content is a very important feature in the adsorption capacity. ► The process is favored under low pH conditions. ► Role of the iron oxide and aluminium oxide in the phosphate adsorption.