| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 149539 | Chemical Engineering Journal | 2012 | 11 Pages |
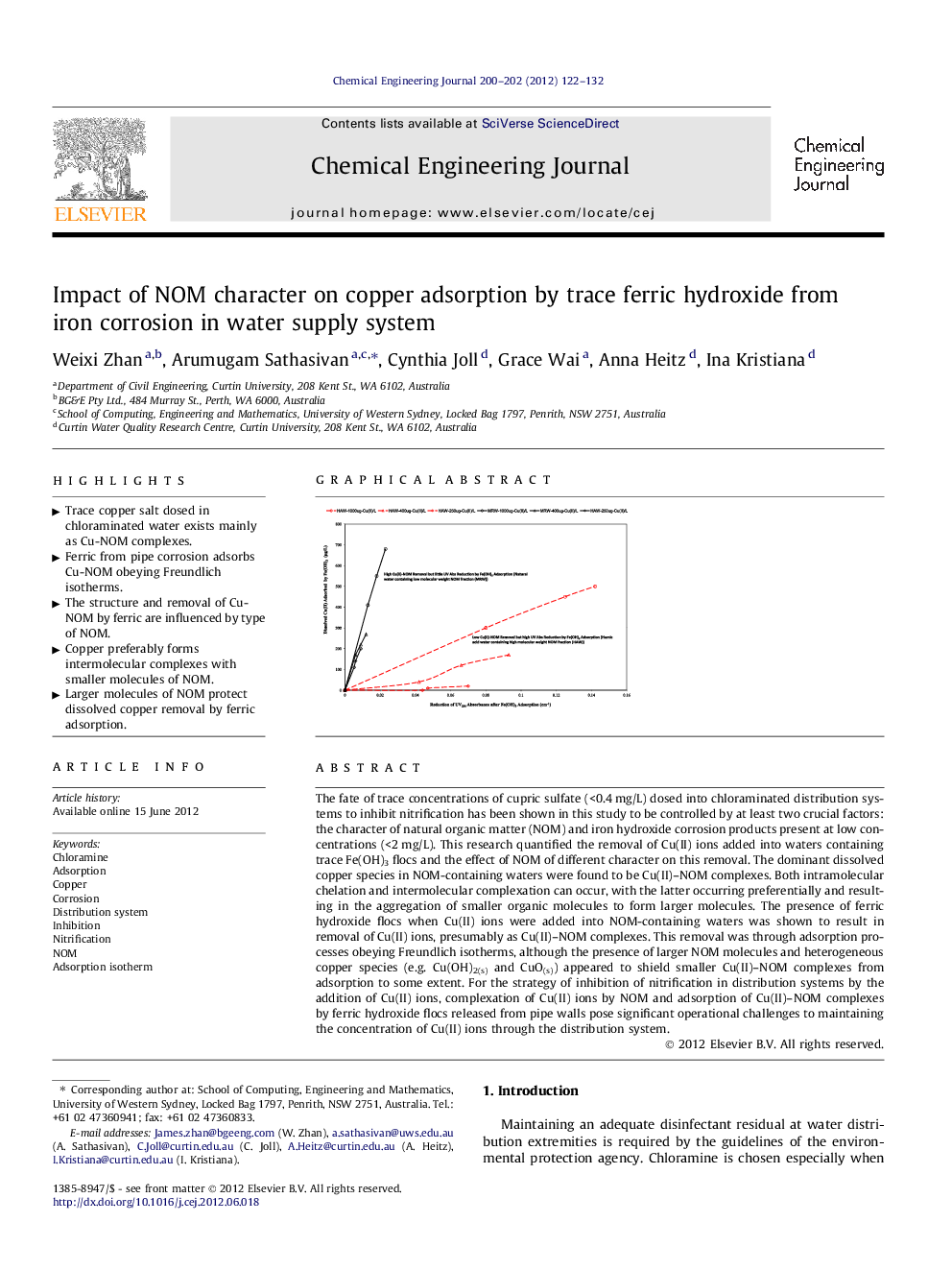
The fate of trace concentrations of cupric sulfate (<0.4 mg/L) dosed into chloraminated distribution systems to inhibit nitrification has been shown in this study to be controlled by at least two crucial factors: the character of natural organic matter (NOM) and iron hydroxide corrosion products present at low concentrations (<2 mg/L). This research quantified the removal of Cu(II) ions added into waters containing trace Fe(OH)3 flocs and the effect of NOM of different character on this removal. The dominant dissolved copper species in NOM-containing waters were found to be Cu(II)–NOM complexes. Both intramolecular chelation and intermolecular complexation can occur, with the latter occurring preferentially and resulting in the aggregation of smaller organic molecules to form larger molecules. The presence of ferric hydroxide flocs when Cu(II) ions were added into NOM-containing waters was shown to result in removal of Cu(II) ions, presumably as Cu(II)–NOM complexes. This removal was through adsorption processes obeying Freundlich isotherms, although the presence of larger NOM molecules and heterogeneous copper species (e.g. Cu(OH)2(s) and CuO(s)) appeared to shield smaller Cu(II)–NOM complexes from adsorption to some extent. For the strategy of inhibition of nitrification in distribution systems by the addition of Cu(II) ions, complexation of Cu(II) ions by NOM and adsorption of Cu(II)–NOM complexes by ferric hydroxide flocs released from pipe walls pose significant operational challenges to maintaining the concentration of Cu(II) ions through the distribution system.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Trace copper salt dosed in chloraminated water exists mainly as Cu-NOM complexes. ► Ferric from pipe corrosion adsorbs Cu-NOM obeying Freundlich isotherms. ► The structure and removal of Cu-NOM by ferric are influenced by type of NOM. ► Copper preferably forms intermolecular complexes with smaller molecules of NOM. ► Larger molecules of NOM protect dissolved copper removal by ferric adsorption.