| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 149598 | Chemical Engineering Journal | 2012 | 10 Pages |
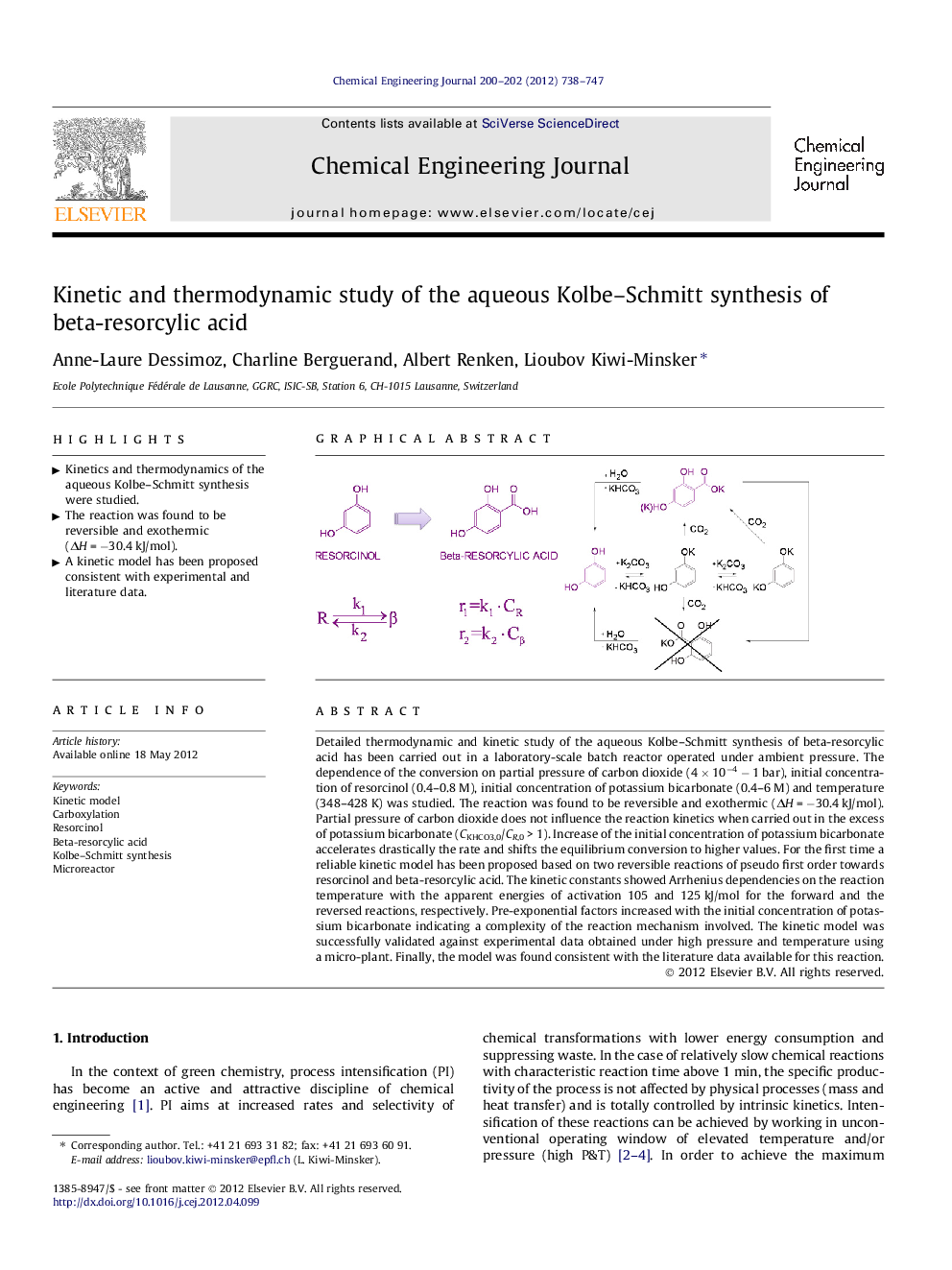
Detailed thermodynamic and kinetic study of the aqueous Kolbe–Schmitt synthesis of beta-resorcylic acid has been carried out in a laboratory-scale batch reactor operated under ambient pressure. The dependence of the conversion on partial pressure of carbon dioxide (4 × 10−4 − 1 bar), initial concentration of resorcinol (0.4–0.8 M), initial concentration of potassium bicarbonate (0.4–6 M) and temperature (348–428 K) was studied. The reaction was found to be reversible and exothermic (ΔH = −30.4 kJ/mol). Partial pressure of carbon dioxide does not influence the reaction kinetics when carried out in the excess of potassium bicarbonate (CKHCO3,0/CR,0 > 1). Increase of the initial concentration of potassium bicarbonate accelerates drastically the rate and shifts the equilibrium conversion to higher values. For the first time a reliable kinetic model has been proposed based on two reversible reactions of pseudo first order towards resorcinol and beta-resorcylic acid. The kinetic constants showed Arrhenius dependencies on the reaction temperature with the apparent energies of activation 105 and 125 kJ/mol for the forward and the reversed reactions, respectively. Pre-exponential factors increased with the initial concentration of potassium bicarbonate indicating a complexity of the reaction mechanism involved. The kinetic model was successfully validated against experimental data obtained under high pressure and temperature using a micro-plant. Finally, the model was found consistent with the literature data available for this reaction.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Kinetics and thermodynamics of the aqueous Kolbe–Schmitt synthesis were studied. ► The reaction was found to be reversible and exothermic (ΔH = −30.4 kJ/mol). ► A kinetic model has been proposed consistent with experimental and literature data.