| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 149630 | Chemical Engineering Journal | 2012 | 10 Pages |
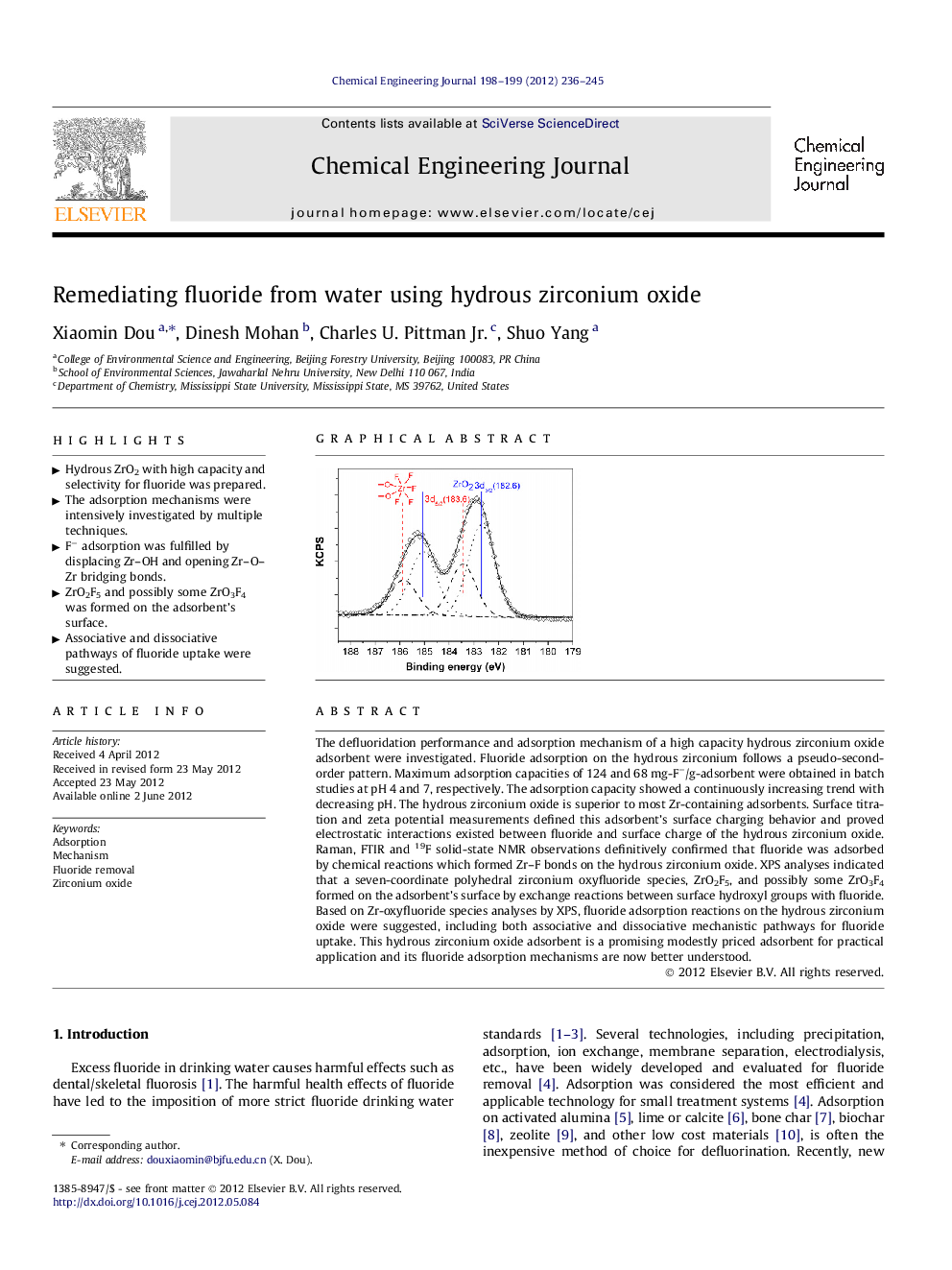
The defluoridation performance and adsorption mechanism of a high capacity hydrous zirconium oxide adsorbent were investigated. Fluoride adsorption on the hydrous zirconium follows a pseudo-second-order pattern. Maximum adsorption capacities of 124 and 68 mg-F−/g-adsorbent were obtained in batch studies at pH 4 and 7, respectively. The adsorption capacity showed a continuously increasing trend with decreasing pH. The hydrous zirconium oxide is superior to most Zr-containing adsorbents. Surface titration and zeta potential measurements defined this adsorbent’s surface charging behavior and proved electrostatic interactions existed between fluoride and surface charge of the hydrous zirconium oxide. Raman, FTIR and 19F solid-state NMR observations definitively confirmed that fluoride was adsorbed by chemical reactions which formed Zr–F bonds on the hydrous zirconium oxide. XPS analyses indicated that a seven-coordinate polyhedral zirconium oxyfluoride species, ZrO2F5, and possibly some ZrO3F4 formed on the adsorbent’s surface by exchange reactions between surface hydroxyl groups with fluoride. Based on Zr-oxyfluoride species analyses by XPS, fluoride adsorption reactions on the hydrous zirconium oxide were suggested, including both associative and dissociative mechanistic pathways for fluoride uptake. This hydrous zirconium oxide adsorbent is a promising modestly priced adsorbent for practical application and its fluoride adsorption mechanisms are now better understood.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Hydrous ZrO2 with high capacity and selectivity for fluoride was prepared. ► The adsorption mechanisms were intensively investigated by multiple techniques. ► F− adsorption was fulfilled by displacing Zr–OH and opening Zr–O–Zr bridging bonds. ► ZrO2F5 and possibly some ZrO3F4 was formed on the adsorbent’s surface. ► Associative and dissociative pathways of fluoride uptake were suggested.