| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 149644 | Chemical Engineering Journal | 2012 | 9 Pages |
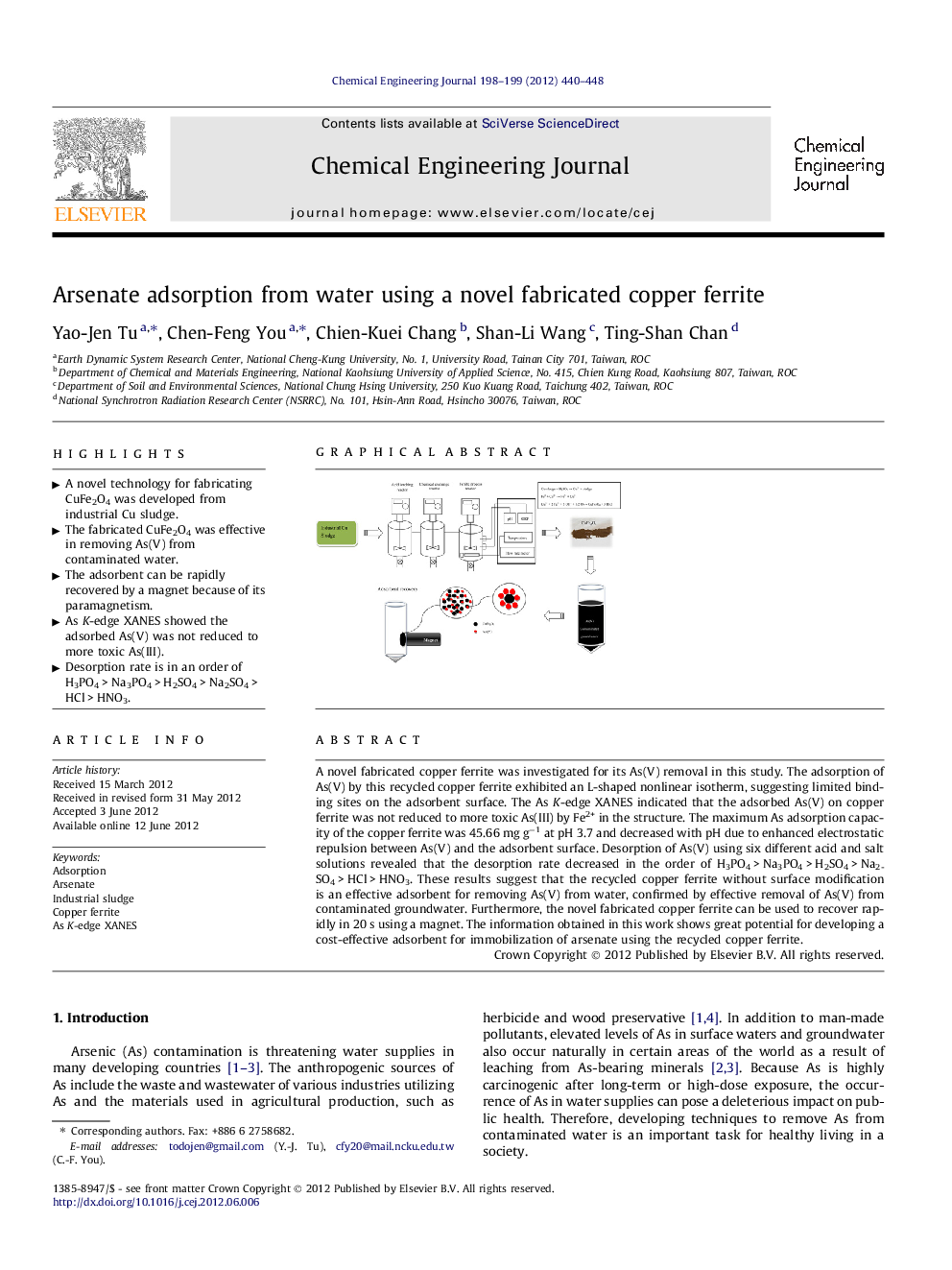
A novel fabricated copper ferrite was investigated for its As(V) removal in this study. The adsorption of As(V) by this recycled copper ferrite exhibited an L-shaped nonlinear isotherm, suggesting limited binding sites on the adsorbent surface. The As K-edge XANES indicated that the adsorbed As(V) on copper ferrite was not reduced to more toxic As(III) by Fe2+ in the structure. The maximum As adsorption capacity of the copper ferrite was 45.66 mg g−1 at pH 3.7 and decreased with pH due to enhanced electrostatic repulsion between As(V) and the adsorbent surface. Desorption of As(V) using six different acid and salt solutions revealed that the desorption rate decreased in the order of H3PO4 > Na3PO4 > H2SO4 > Na2SO4 > HCl > HNO3. These results suggest that the recycled copper ferrite without surface modification is an effective adsorbent for removing As(V) from water, confirmed by effective removal of As(V) from contaminated groundwater. Furthermore, the novel fabricated copper ferrite can be used to recover rapidly in 20 s using a magnet. The information obtained in this work shows great potential for developing a cost-effective adsorbent for immobilization of arsenate using the recycled copper ferrite.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► A novel technology for fabricating CuFe2O4 was developed from industrial Cu sludge. ► The fabricated CuFe2O4 was effective in removing As(V) from contaminated water. ► The adsorbent can be rapidly recovered by a magnet because of its paramagnetism. ► As K-edge XANES showed the adsorbed As(V) was not reduced to more toxic As(III). ► Desorption rate is in an order of H3PO4 > Na3PO4 > H2SO4 > Na2SO4 > HCl > HNO3.