| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 149667 | Chemical Engineering Journal | 2012 | 11 Pages |
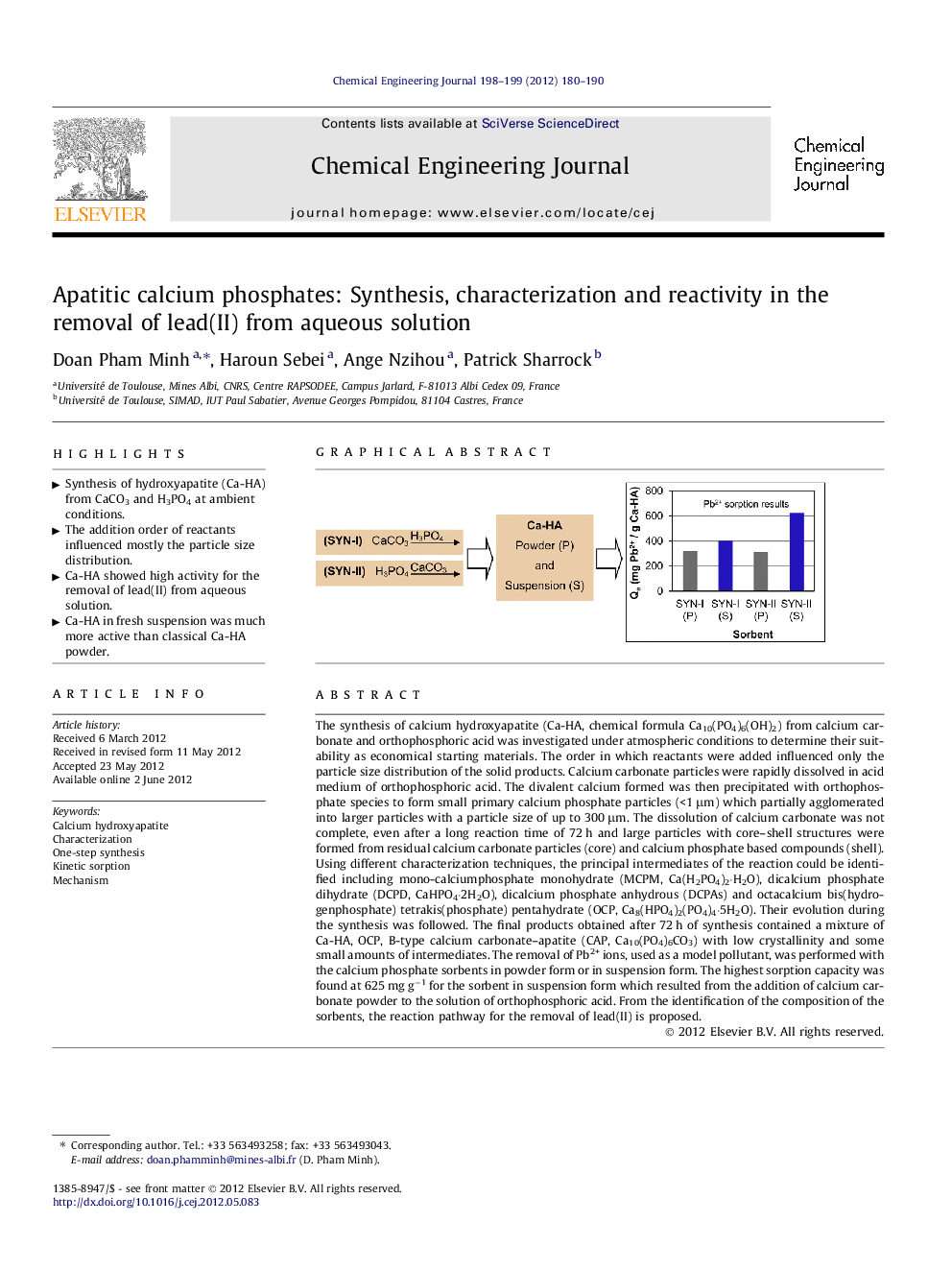
The synthesis of calcium hydroxyapatite (Ca-HA, chemical formula Ca10(PO4)6(OH)2) from calcium carbonate and orthophosphoric acid was investigated under atmospheric conditions to determine their suitability as economical starting materials. The order in which reactants were added influenced only the particle size distribution of the solid products. Calcium carbonate particles were rapidly dissolved in acid medium of orthophosphoric acid. The divalent calcium formed was then precipitated with orthophosphate species to form small primary calcium phosphate particles (<1 μm) which partially agglomerated into larger particles with a particle size of up to 300 μm. The dissolution of calcium carbonate was not complete, even after a long reaction time of 72 h and large particles with core–shell structures were formed from residual calcium carbonate particles (core) and calcium phosphate based compounds (shell). Using different characterization techniques, the principal intermediates of the reaction could be identified including mono-calciumphosphate monohydrate (MCPM, Ca(H2PO4)2·H2O), dicalcium phosphate dihydrate (DCPD, CaHPO4·2H2O), dicalcium phosphate anhydrous (DCPAs) and octacalcium bis(hydrogenphosphate) tetrakis(phosphate) pentahydrate (OCP, Ca8(HPO4)2(PO4)4·5H2O). Their evolution during the synthesis was followed. The final products obtained after 72 h of synthesis contained a mixture of Ca-HA, OCP, B-type calcium carbonate–apatite (CAP, Ca10(PO4)6CO3) with low crystallinity and some small amounts of intermediates. The removal of Pb2+ ions, used as a model pollutant, was performed with the calcium phosphate sorbents in powder form or in suspension form. The highest sorption capacity was found at 625 mg g−1 for the sorbent in suspension form which resulted from the addition of calcium carbonate powder to the solution of orthophosphoric acid. From the identification of the composition of the sorbents, the reaction pathway for the removal of lead(II) is proposed.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Synthesis of hydroxyapatite (Ca-HA) from CaCO3 and H3PO4 at ambient conditions. ► The addition order of reactants influenced mostly the particle size distribution. ► Ca-HA showed high activity for the removal of lead(II) from aqueous solution. ► Ca-HA in fresh suspension was much more active than classical Ca-HA powder.