| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 149698 | Chemical Engineering Journal | 2012 | 11 Pages |
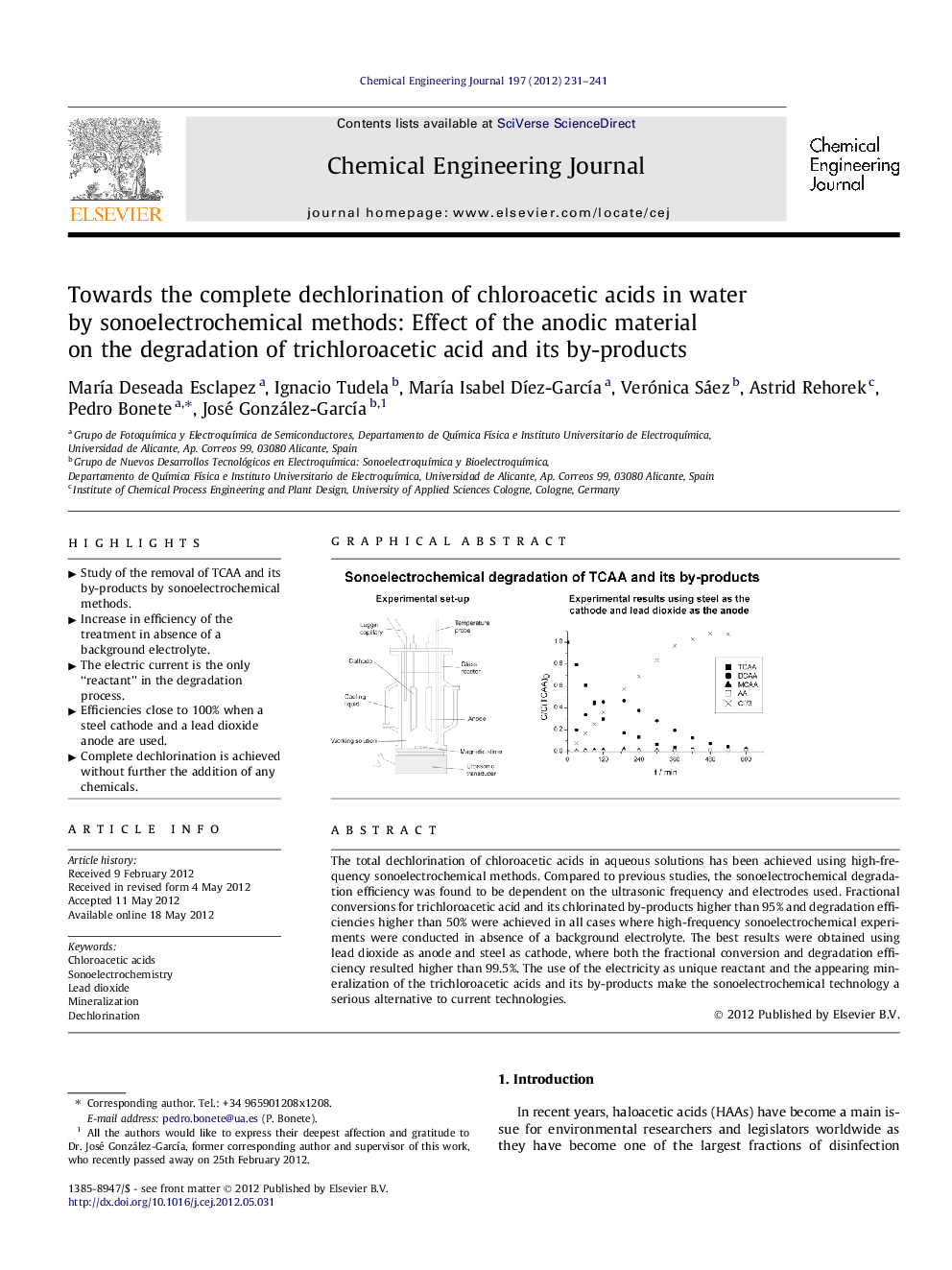
The total dechlorination of chloroacetic acids in aqueous solutions has been achieved using high-frequency sonoelectrochemical methods. Compared to previous studies, the sonoelectrochemical degradation efficiency was found to be dependent on the ultrasonic frequency and electrodes used. Fractional conversions for trichloroacetic acid and its chlorinated by-products higher than 95% and degradation efficiencies higher than 50% were achieved in all cases where high-frequency sonoelectrochemical experiments were conducted in absence of a background electrolyte. The best results were obtained using lead dioxide as anode and steel as cathode, where both the fractional conversion and degradation efficiency resulted higher than 99.5%. The use of the electricity as unique reactant and the appearing mineralization of the trichloroacetic acids and its by-products make the sonoelectrochemical technology a serious alternative to current technologies.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlights► Study of the removal of TCAA and its by-products by sonoelectrochemical methods. ► Increase in efficiency of the treatment in absence of a background electrolyte. ► The electric current is the only “reactant” in the degradation process. ► Efficiencies close to 100% when a steel cathode and a lead dioxide anode are used. ► Complete dechlorination is achieved without further the addition of any chemicals.