| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 149736 | Chemical Engineering Journal | 2012 | 9 Pages |
The high selectivity for the separation of nickel from cobalt-solution by a polyamine chelating resin was explored. As to the sole-component static system, the equilibrium data could be satisfactorily described by the Langmuir isotherm, from which the calculated maximum adsorption capacities for Ni(II) and Co(II) were 0.982 mmol/g and 0.741 mmol/g, respectively. Additionally, in the binary system with high concentration ratio of cobalt and nickel, the uptake amount of resin for both ions decreased, indicating their competitive adsorption behavior on active sites. Separation factors suggested the extremely higher selectivity for Ni(II) against Co(II). The obtained separation factor values indicated the extremely higher selectivity for Ni(II) versus Co(II). The column dynamic breakthrough curves revealed a potential success for the achievement of high-purity cobalt because of the higher initial adsorption rate and capacity toward Ni(II).
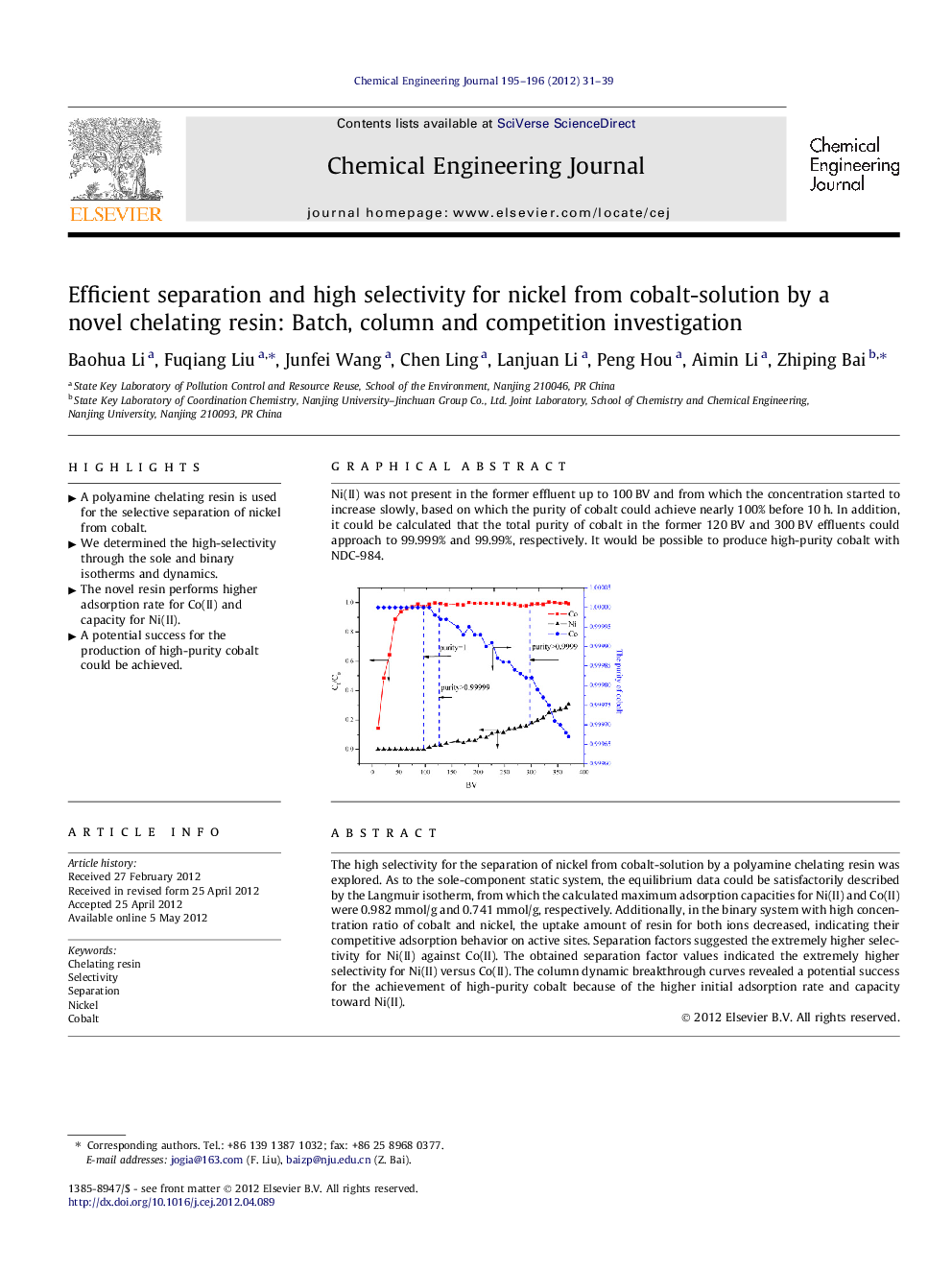
Graphical abstractNi(II) was not present in the former effluent up to 100 BV and from which the concentration started to increase slowly, based on which the purity of cobalt could achieve nearly 100% before 10 h. In addition, it could be calculated that the total purity of cobalt in the former 120 BV and 300 BV effluents could approach to 99.999% and 99.99%, respectively. It would be possible to produce high-purity cobalt with NDC-984.Figure optionsDownload full-size imageDownload as PowerPoint slideHighlight► A polyamine chelating resin is used for the selective separation of nickel from cobalt. ► We determined the high-selectivity through the sole and binary isotherms and dynamics. ► The novel resin performs higher adsorption rate for Co(II) and capacity for Ni(II). ► A potential success for the production of high-purity cobalt could be achieved.