| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 149761 | Chemical Engineering Journal | 2012 | 8 Pages |
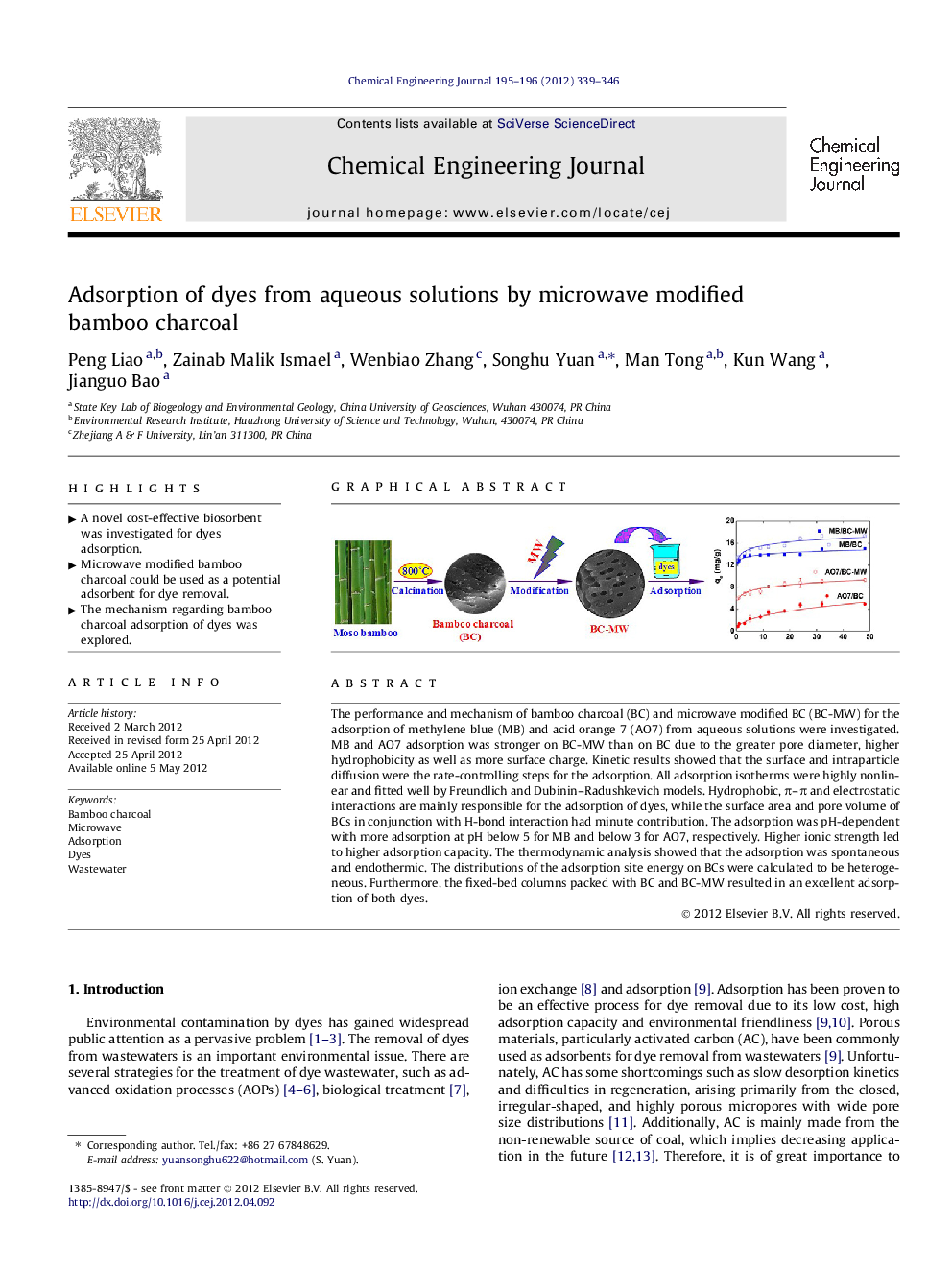
The performance and mechanism of bamboo charcoal (BC) and microwave modified BC (BC-MW) for the adsorption of methylene blue (MB) and acid orange 7 (AO7) from aqueous solutions were investigated. MB and AO7 adsorption was stronger on BC-MW than on BC due to the greater pore diameter, higher hydrophobicity as well as more surface charge. Kinetic results showed that the surface and intraparticle diffusion were the rate-controlling steps for the adsorption. All adsorption isotherms were highly nonlinear and fitted well by Freundlich and Dubinin–Radushkevich models. Hydrophobic, π–π and electrostatic interactions are mainly responsible for the adsorption of dyes, while the surface area and pore volume of BCs in conjunction with H-bond interaction had minute contribution. The adsorption was pH-dependent with more adsorption at pH below 5 for MB and below 3 for AO7, respectively. Higher ionic strength led to higher adsorption capacity. The thermodynamic analysis showed that the adsorption was spontaneous and endothermic. The distributions of the adsorption site energy on BCs were calculated to be heterogeneous. Furthermore, the fixed-bed columns packed with BC and BC-MW resulted in an excellent adsorption of both dyes.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlight► A novel cost-effective biosorbent was investigated for dyes adsorption. ► Microwave modified bamboo charcoal could be used as a potential adsorbent for dye removal. ► The mechanism regarding bamboo charcoal adsorption of dyes was explored..