| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 149852 | Chemical Engineering Journal | 2012 | 11 Pages |
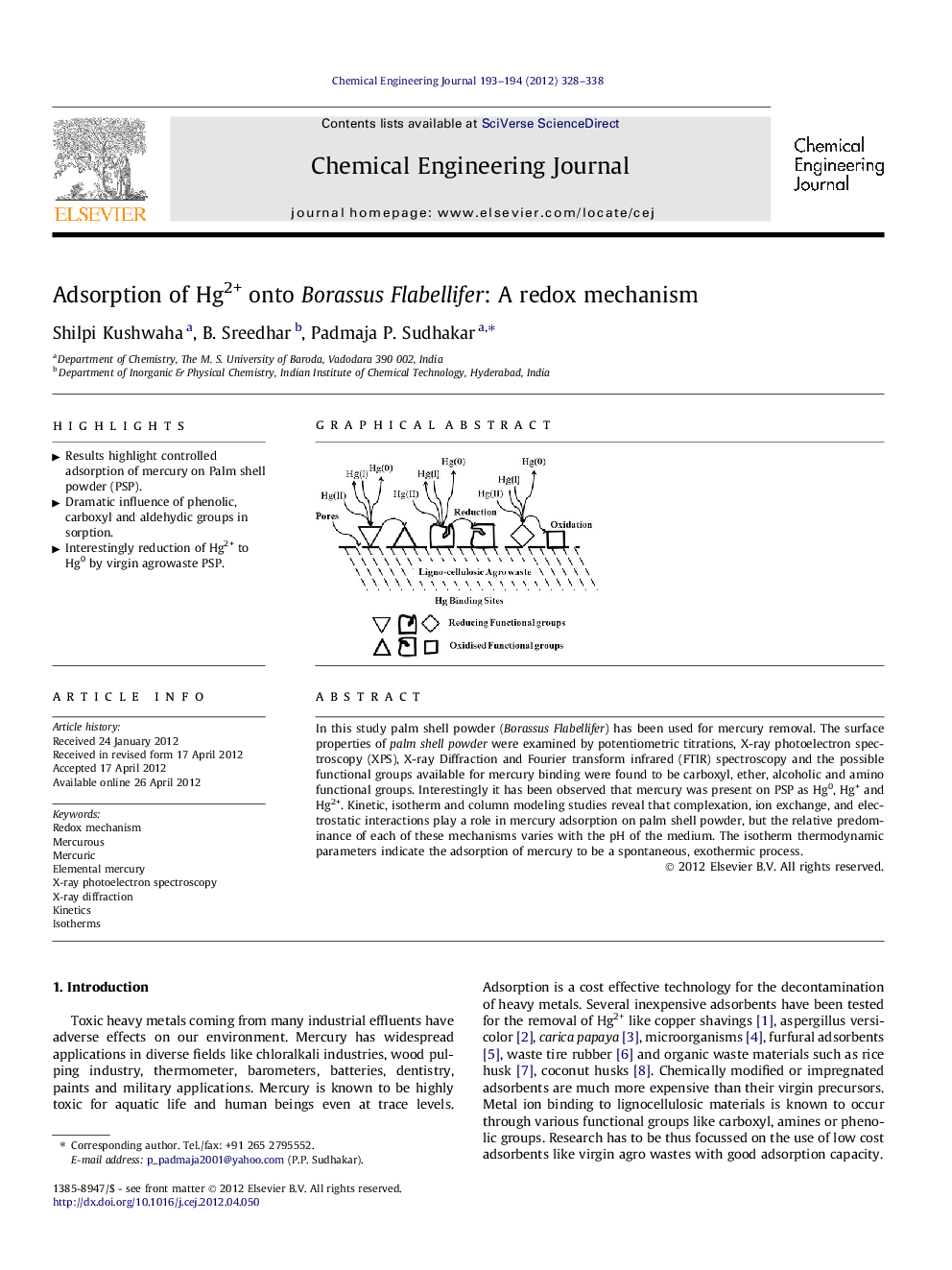
In this study palm shell powder (Borassus Flabellifer) has been used for mercury removal. The surface properties of palm shell powder were examined by potentiometric titrations, X-ray photoelectron spectroscopy (XPS), X-ray Diffraction and Fourier transform infrared (FTIR) spectroscopy and the possible functional groups available for mercury binding were found to be carboxyl, ether, alcoholic and amino functional groups. Interestingly it has been observed that mercury was present on PSP as Hg0, Hg+ and Hg2+. Kinetic, isotherm and column modeling studies reveal that complexation, ion exchange, and electrostatic interactions play a role in mercury adsorption on palm shell powder, but the relative predominance of each of these mechanisms varies with the pH of the medium. The isotherm thermodynamic parameters indicate the adsorption of mercury to be a spontaneous, exothermic process.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slideHighlight► Results highlight controlled adsorption of mercury on Palm shell powder (PSP). ► Dramatic influence of phenolic, carboxyl and aldehydic groups in sorption. ► Interestingly reduction of Hg2+ to Hg0 by virgin agrowaste PSP.