| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 2039636 | Cell Reports | 2017 | 7 Pages |
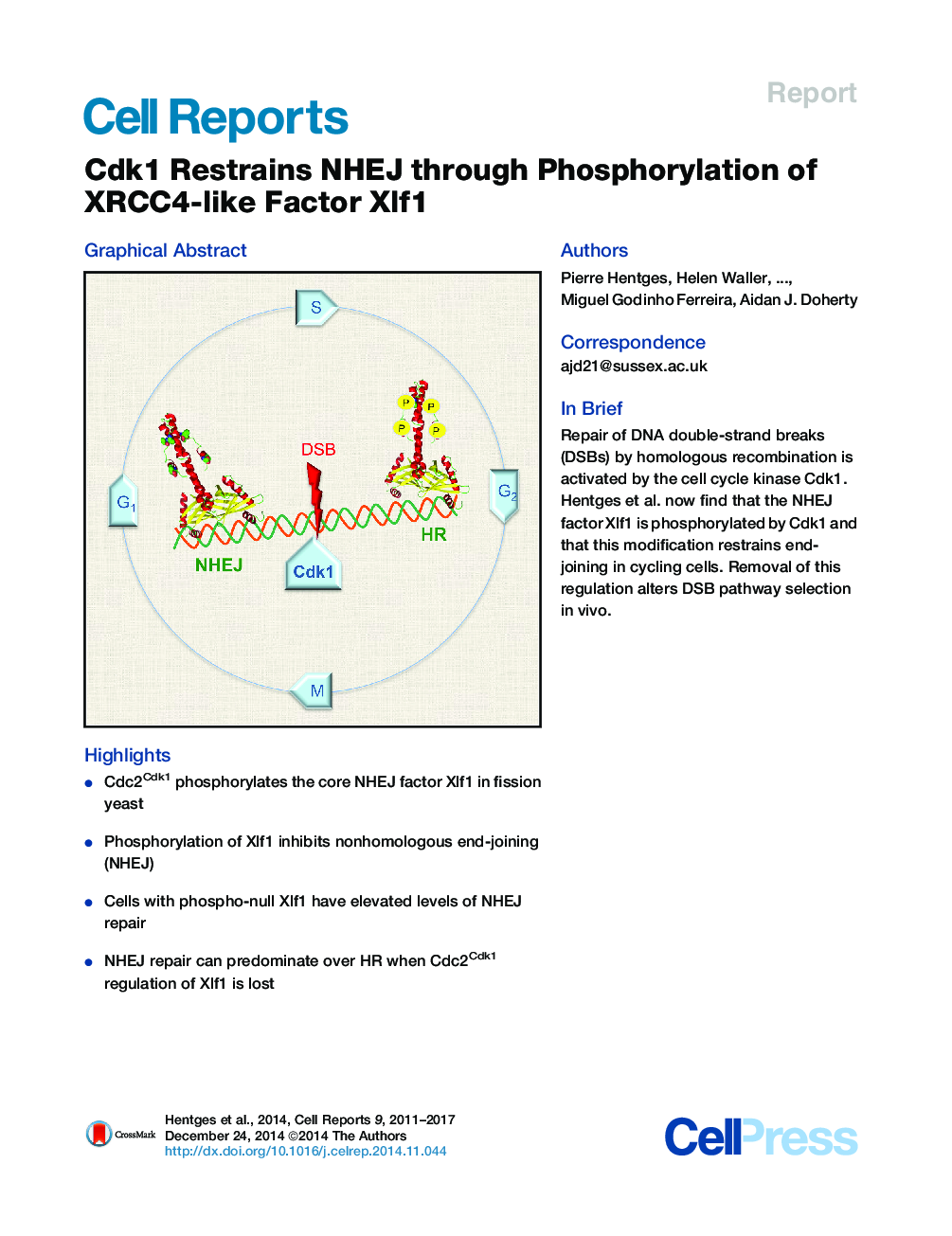
•Cdc2Cdk1 phosphorylates the core NHEJ factor Xlf1 in fission yeast•Phosphorylation of Xlf1 inhibits nonhomologous end-joining (NHEJ)•Cells with phospho-null Xlf1 have elevated levels of NHEJ repair•NHEJ repair can predominate over HR when Cdc2Cdk1 regulation of Xlf1 is lost
SummaryEukaryotic cells use two principal mechanisms for repairing DNA double-strand breaks (DSBs): homologous recombination (HR) and nonhomologous end-joining (NHEJ). DSB repair pathway choice is strongly regulated during the cell cycle. Cyclin-dependent kinase 1 (Cdk1) activates HR by phosphorylation of key recombination factors. However, a mechanism for regulating the NHEJ pathway has not been established. Here, we report that Xlf1, a fission yeast XLF ortholog, is a key regulator of NHEJ activity in the cell cycle. We show that Cdk1 phosphorylates residues in the C terminus of Xlf1 over the course of the cell cycle. Mutation of these residues leads to the loss of Cdk1 phosphorylation, resulting in elevated levels of NHEJ repair in vivo. Together, these data establish that Xlf1 phosphorylation by Cdc2Cdk1 provides a molecular mechanism for downregulation of NHEJ in fission yeast and indicates that XLF is a key regulator of end-joining processes in eukaryotic organisms.
Graphical AbstractFigure optionsDownload full-size imageDownload as PowerPoint slide