| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 6465081 | Chemical Engineering Journal | 2017 | 13 Pages |
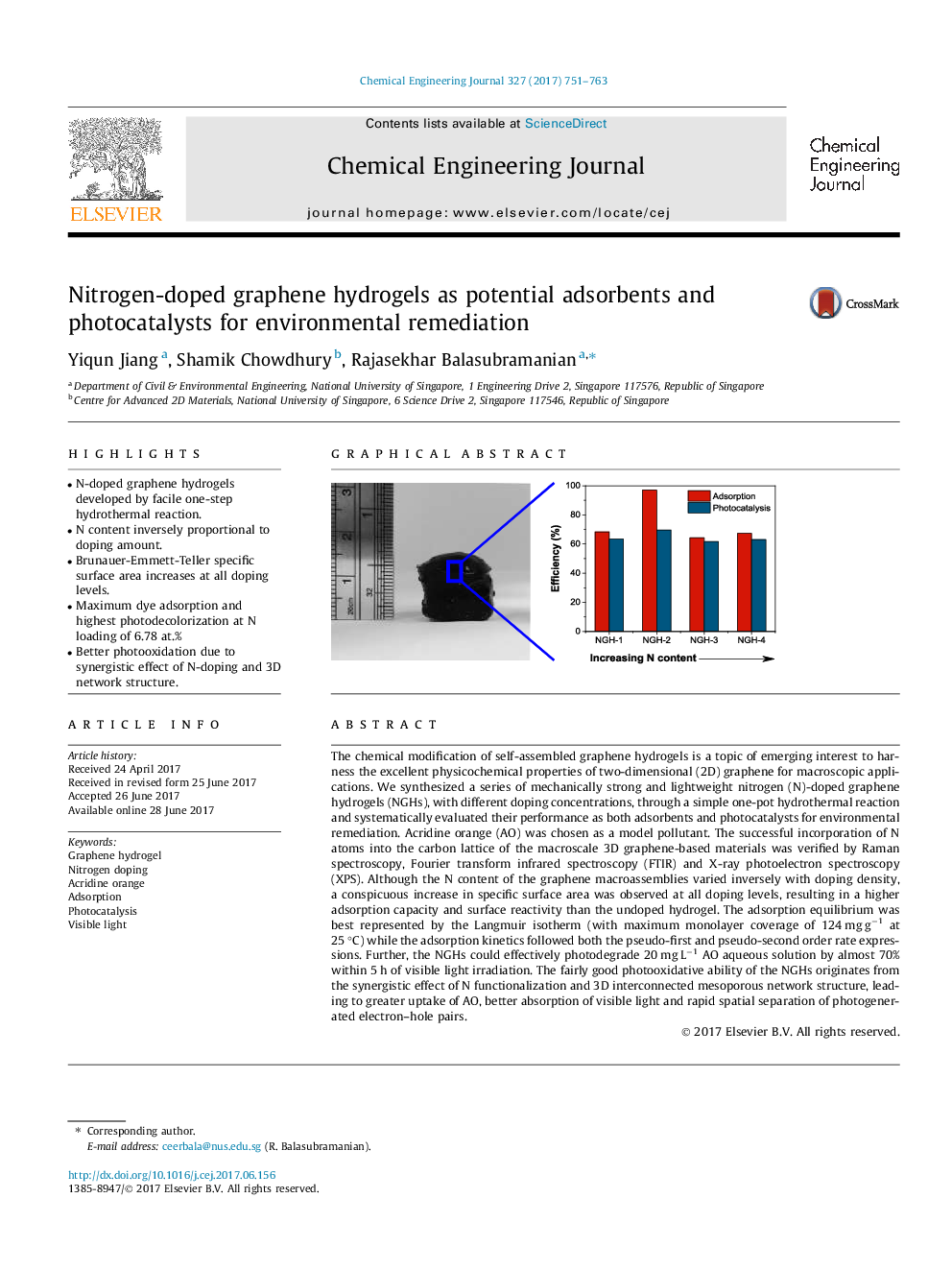
â¢N-doped graphene hydrogels developed by facile one-step hydrothermal reaction.â¢N content inversely proportional to doping amount.â¢Brunauer-Emmett-Teller specific surface area increases at all doping levels.â¢Maximum dye adsorption and highest photodecolorization at N loading of 6.78 at.%â¢Better photooxidation due to synergistic effect of N-doping and 3D network structure.
The chemical modification of self-assembled graphene hydrogels is a topic of emerging interest to harness the excellent physicochemical properties of two-dimensional (2D) graphene for macroscopic applications. We synthesized a series of mechanically strong and lightweight nitrogen (N)-doped graphene hydrogels (NGHs), with different doping concentrations, through a simple one-pot hydrothermal reaction and systematically evaluated their performance as both adsorbents and photocatalysts for environmental remediation. Acridine orange (AO) was chosen as a model pollutant. The successful incorporation of N atoms into the carbon lattice of the macroscale 3D graphene-based materials was verified by Raman spectroscopy, Fourier transform infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS). Although the N content of the graphene macroassemblies varied inversely with doping density, a conspicuous increase in specific surface area was observed at all doping levels, resulting in a higher adsorption capacity and surface reactivity than the undoped hydrogel. The adsorption equilibrium was best represented by the Langmuir isotherm (with maximum monolayer coverage of 124 mg gâ1 at 25 °C) while the adsorption kinetics followed both the pseudo-first and pseudo-second order rate expressions. Further, the NGHs could effectively photodegrade 20 mg Lâ1 AO aqueous solution by almost 70% within 5 h of visible light irradiation. The fairly good photooxidative ability of the NGHs originates from the synergistic effect of N functionalization and 3D interconnected mesoporous network structure, leading to greater uptake of AO, better absorption of visible light and rapid spatial separation of photogenerated electron-hole pairs.
Graphical abstractDownload high-res image (147KB)Download full-size image