| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 6465156 | Chemical Engineering Journal | 2017 | 11 Pages |
â¢Acid carbon is an attractive alternative for hydrocracking catalyst supports.â¢High reaction temperature attenuates catalyst deactivation but favors coke aging.â¢TPO reveals the presence of light deactivating species on the catalyst surface.â¢LDI/MS uncovers the nature of deactivating species deposited on carbon.â¢Catalyst properties are partially recovered through controlled-combustion.
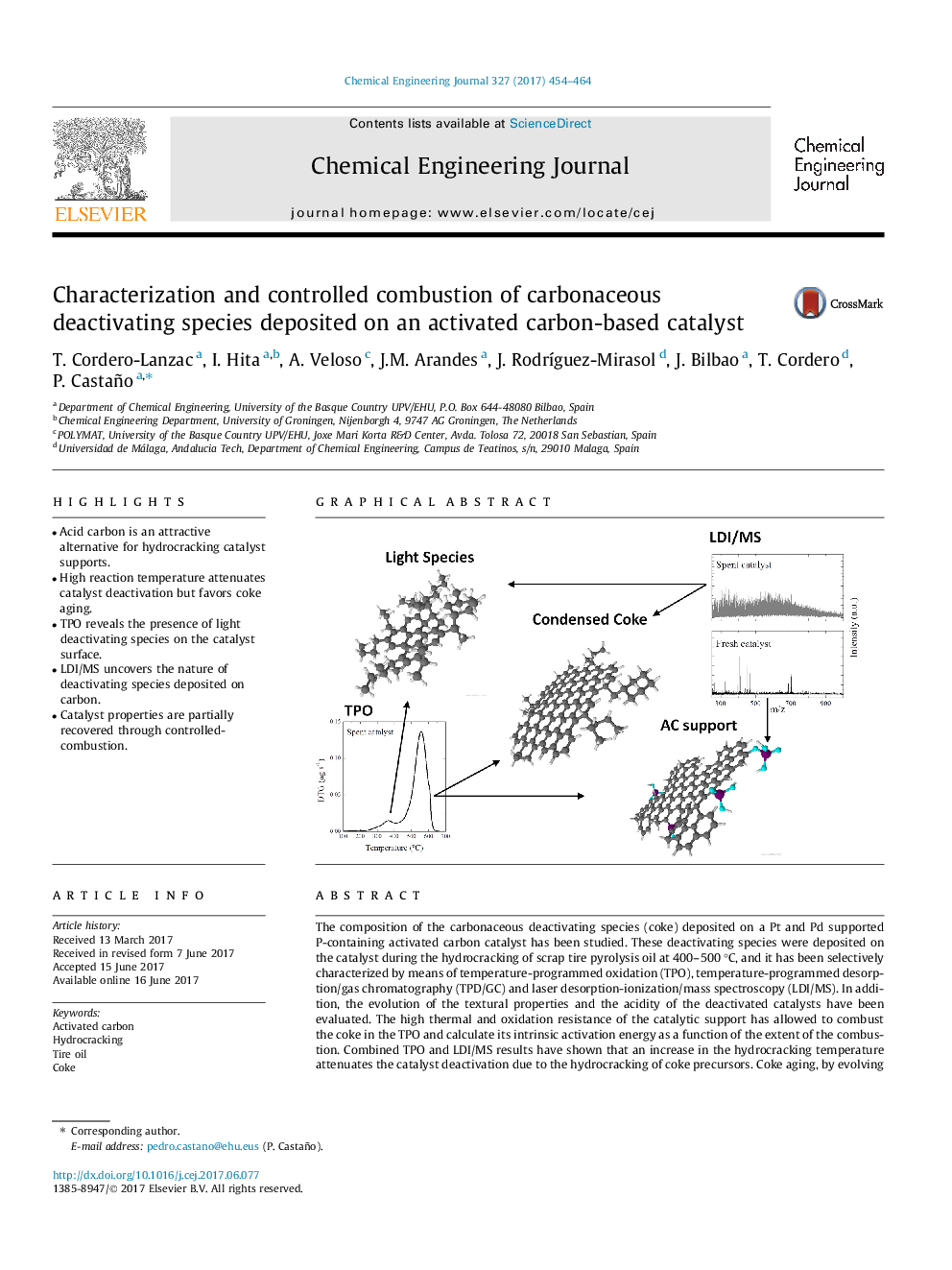
The composition of the carbonaceous deactivating species (coke) deposited on a Pt and Pd supported P-containing activated carbon catalyst has been studied. These deactivating species were deposited on the catalyst during the hydrocracking of scrap tire pyrolysis oil at 400-500 °C, and it has been selectively characterized by means of temperature-programmed oxidation (TPO), temperature-programmed desorption/gas chromatography (TPD/GC) and laser desorption-ionization/mass spectroscopy (LDI/MS). In addition, the evolution of the textural properties and the acidity of the deactivated catalysts have been evaluated. The high thermal and oxidation resistance of the catalytic support has allowed to combust the coke in the TPO and calculate its intrinsic activation energy as a function of the extent of the combustion. Combined TPO and LDI/MS results have shown that an increase in the hydrocracking temperature attenuates the catalyst deactivation due to the hydrocracking of coke precursors. Coke aging, by evolving towards a more condensed structure, is also favored at higher hydrocracking temperatures. The combustion of the most condensed coke requires of higher temperatures than 375 °C, which hinders the complete regeneration of the activated carbon-based catalyst.
Graphical abstractDownload high-res image (130KB)Download full-size image