| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 6465226 | Chemical Engineering Journal | 2017 | 8 Pages |
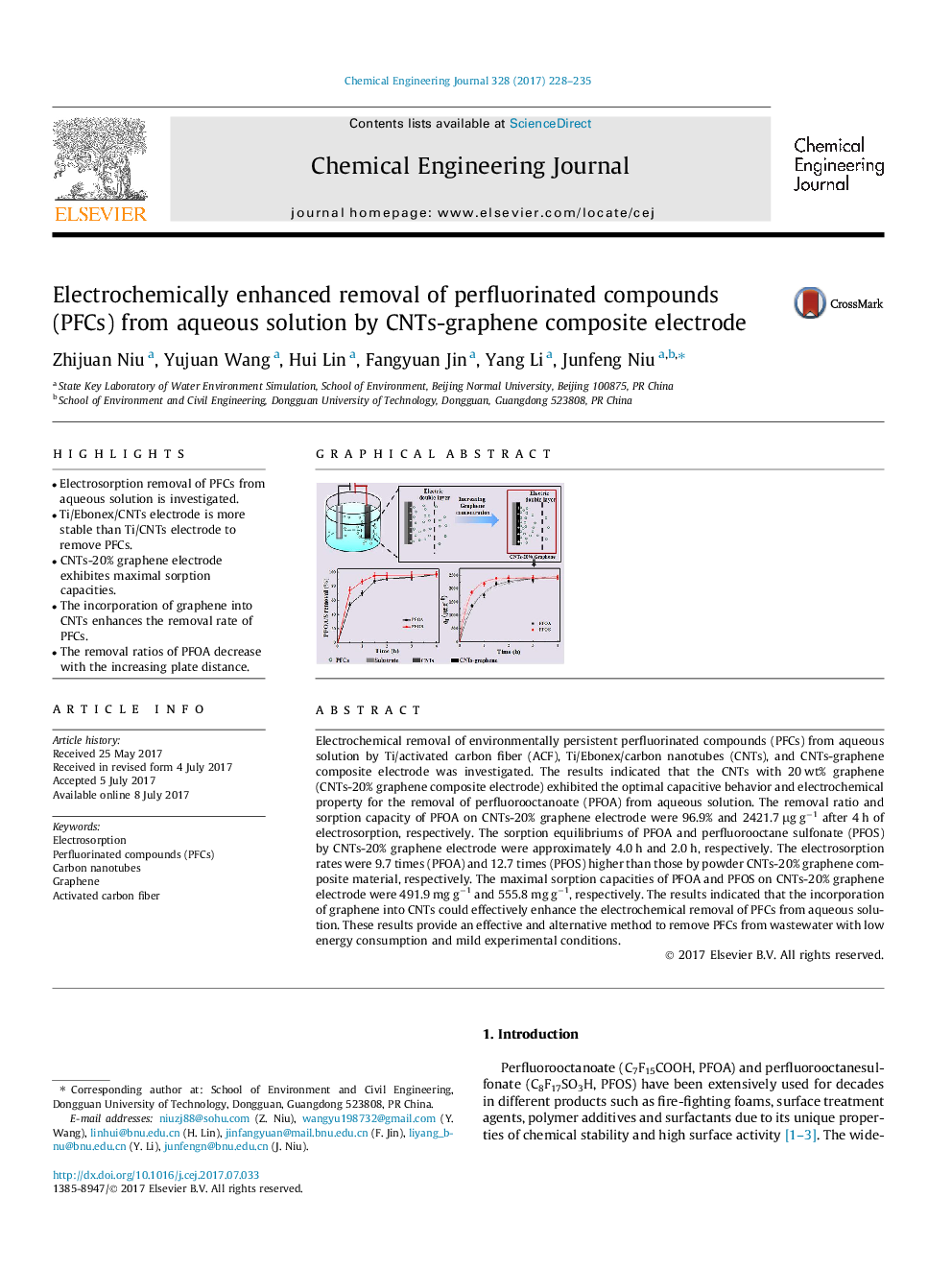
â¢Electrosorption removal of PFCs from aqueous solution is investigated.â¢Ti/Ebonex/CNTs electrode is more stable than Ti/CNTs electrode to remove PFCs.â¢CNTs-20% graphene electrode exhibites maximal sorption capacities.â¢The incorporation of graphene into CNTs enhances the removal rate of PFCs.â¢The removal ratios of PFOA decrease with the increasing plate distance.
Electrochemical removal of environmentally persistent perfluorinated compounds (PFCs) from aqueous solution by Ti/activated carbon fiber (ACF), Ti/Ebonex/carbon nanotubes (CNTs), and CNTs-graphene composite electrode was investigated. The results indicated that the CNTs with 20 wt% graphene (CNTs-20% graphene composite electrode) exhibited the optimal capacitive behavior and electrochemical property for the removal of perfluorooctanoate (PFOA) from aqueous solution. The removal ratio and sorption capacity of PFOA on CNTs-20% graphene electrode were 96.9% and 2421.7 μg gâ1 after 4 h of electrosorption, respectively. The sorption equilibriums of PFOA and perfluorooctane sulfonate (PFOS) by CNTs-20% graphene electrode were approximately 4.0 h and 2.0 h, respectively. The electrosorption rates were 9.7 times (PFOA) and 12.7 times (PFOS) higher than those by powder CNTs-20% graphene composite material, respectively. The maximal sorption capacities of PFOA and PFOS on CNTs-20% graphene electrode were 491.9 mg gâ1 and 555.8 mg gâ1, respectively. The results indicated that the incorporation of graphene into CNTs could effectively enhance the electrochemical removal of PFCs from aqueous solution. These results provide an effective and alternative method to remove PFCs from wastewater with low energy consumption and mild experimental conditions.
Graphical abstractDownload high-res image (65KB)Download full-size image