| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 6465266 | Chemical Engineering Journal | 2017 | 11 Pages |
â¢The sono-assisted preparation of the Fe-Al (30 min) has high adsorption capacity.â¢The pseudo-second-order kinetic model described the experimental data well.â¢The mechanism of uranium removal was investigated.
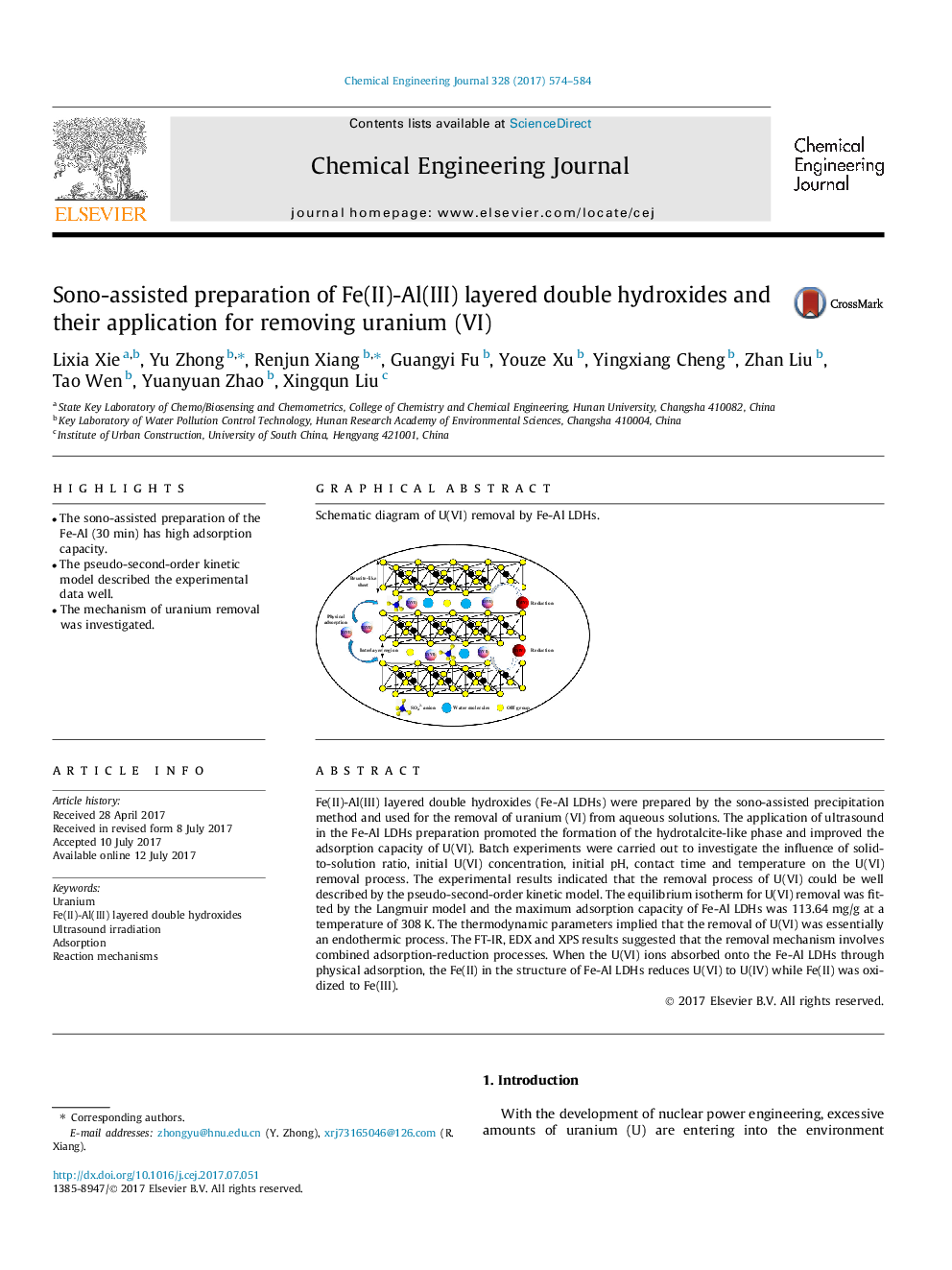
Fe(II)-Al(III) layered double hydroxides (Fe-Al LDHs) were prepared by the sono-assisted precipitation method and used for the removal of uranium (VI) from aqueous solutions. The application of ultrasound in the Fe-Al LDHs preparation promoted the formation of the hydrotalcite-like phase and improved the adsorption capacity of U(VI). Batch experiments were carried out to investigate the influence of solid-to-solution ratio, initial U(VI) concentration, initial pH, contact time and temperature on the U(VI) removal process. The experimental results indicated that the removal process of U(VI) could be well described by the pseudo-second-order kinetic model. The equilibrium isotherm for U(VI) removal was fitted by the Langmuir model and the maximum adsorption capacity of Fe-Al LDHs was 113.64Â mg/g at a temperature of 308Â K. The thermodynamic parameters implied that the removal of U(VI) was essentially an endothermic process. The FT-IR, EDX and XPS results suggested that the removal mechanism involves combined adsorption-reduction processes. When the U(VI) ions absorbed onto the Fe-Al LDHs through physical adsorption, the Fe(II) in the structure of Fe-Al LDHs reduces U(VI) to U(IV) while Fe(II) was oxidized to Fe(III).
Graphical abstractSchematic diagram of U(VI) removal by Fe-Al LDHs.Download high-res image (103KB)Download full-size image