| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 6465305 | Chemical Engineering Journal | 2017 | 9 Pages |
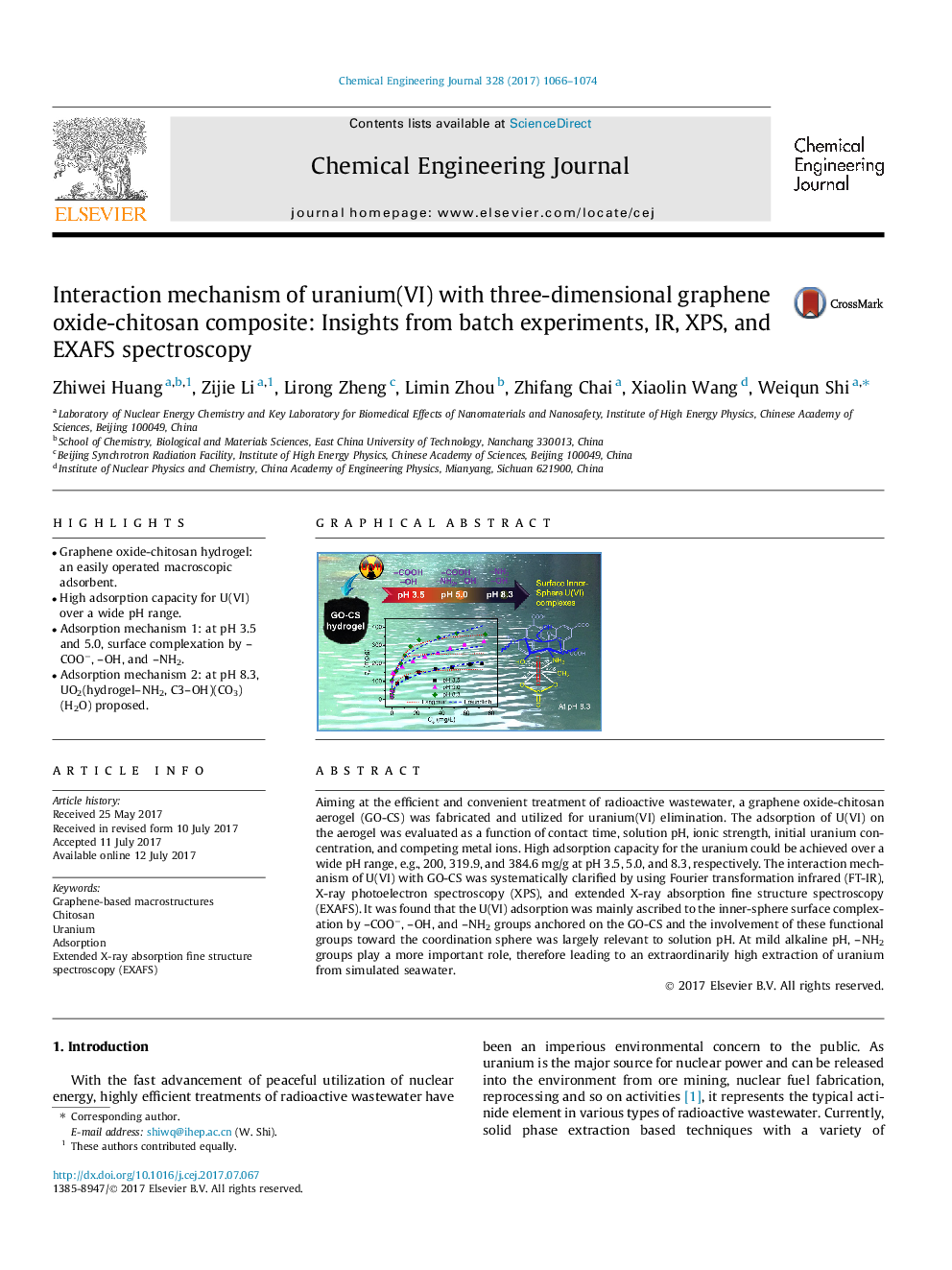
â¢Graphene oxide-chitosan hydrogel: an easily operated macroscopic adsorbent.â¢High adsorption capacity for U(VI) over a wide pH range.â¢Adsorption mechanism 1: at pH 3.5 and 5.0, surface complexation by -COOâ, -OH, and -NH2.â¢Adsorption mechanism 2: at pH 8.3, UO2(hydrogel-NH2, C3-OH)(CO3)(H2O) proposed.
Aiming at the efficient and convenient treatment of radioactive wastewater, a graphene oxide-chitosan aerogel (GO-CS) was fabricated and utilized for uranium(VI) elimination. The adsorption of U(VI) on the aerogel was evaluated as a function of contact time, solution pH, ionic strength, initial uranium concentration, and competing metal ions. High adsorption capacity for the uranium could be achieved over a wide pH range, e.g., 200, 319.9, and 384.6Â mg/g at pH 3.5, 5.0, and 8.3, respectively. The interaction mechanism of U(VI) with GO-CS was systematically clarified by using Fourier transformation infrared (FT-IR), X-ray photoelectron spectroscopy (XPS), and extended X-ray absorption fine structure spectroscopy (EXAFS). It was found that the U(VI) adsorption was mainly ascribed to the inner-sphere surface complexation by -COOâ, -OH, and -NH2 groups anchored on the GO-CS and the involvement of these functional groups toward the coordination sphere was largely relevant to solution pH. At mild alkaline pH, -NH2 groups play a more important role, therefore leading to an extraordinarily high extraction of uranium from simulated seawater.
Graphical abstractDownload high-res image (274KB)Download full-size image