| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 6465315 | Chemical Engineering Journal | 2017 | 6 Pages |
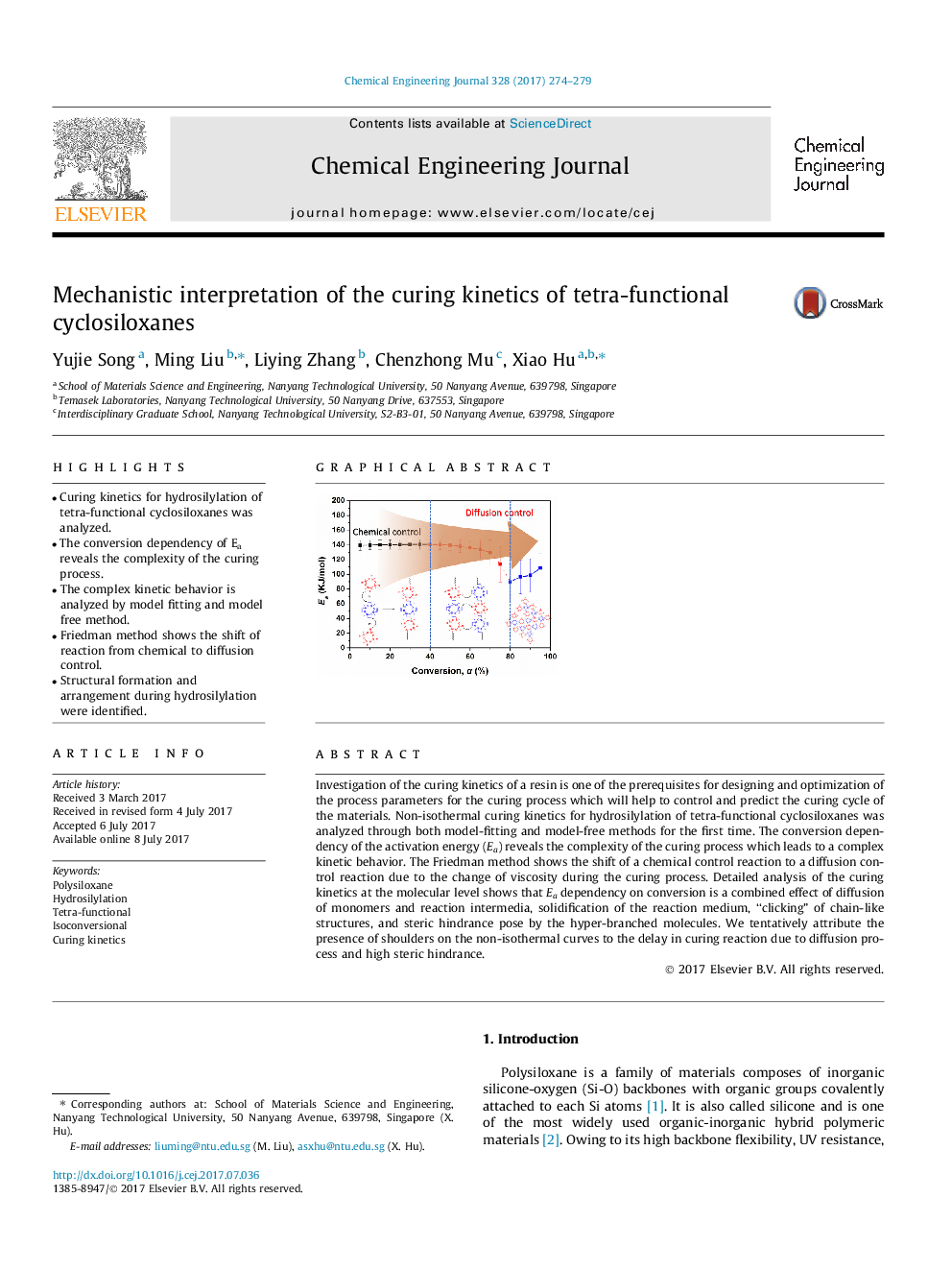
â¢Curing kinetics for hydrosilylation of tetra-functional cyclosiloxanes was analyzed.â¢The conversion dependency of Ea reveals the complexity of the curing process.â¢The complex kinetic behavior is analyzed by model fitting and model free method.â¢Friedman method shows the shift of reaction from chemical to diffusion control.â¢Structural formation and arrangement during hydrosilylation were identified.
Investigation of the curing kinetics of a resin is one of the prerequisites for designing and optimization of the process parameters for the curing process which will help to control and predict the curing cycle of the materials. Non-isothermal curing kinetics for hydrosilylation of tetra-functional cyclosiloxanes was analyzed through both model-fitting and model-free methods for the first time. The conversion dependency of the activation energy (Ea) reveals the complexity of the curing process which leads to a complex kinetic behavior. The Friedman method shows the shift of a chemical control reaction to a diffusion control reaction due to the change of viscosity during the curing process. Detailed analysis of the curing kinetics at the molecular level shows that Ea dependency on conversion is a combined effect of diffusion of monomers and reaction intermedia, solidification of the reaction medium, “clicking” of chain-like structures, and steric hindrance pose by the hyper-branched molecules. We tentatively attribute the presence of shoulders on the non-isothermal curves to the delay in curing reaction due to diffusion process and high steric hindrance.
Graphical abstractDownload high-res image (131KB)Download full-size image