| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 6465487 | Chemical Engineering Journal | 2017 | 8 Pages |
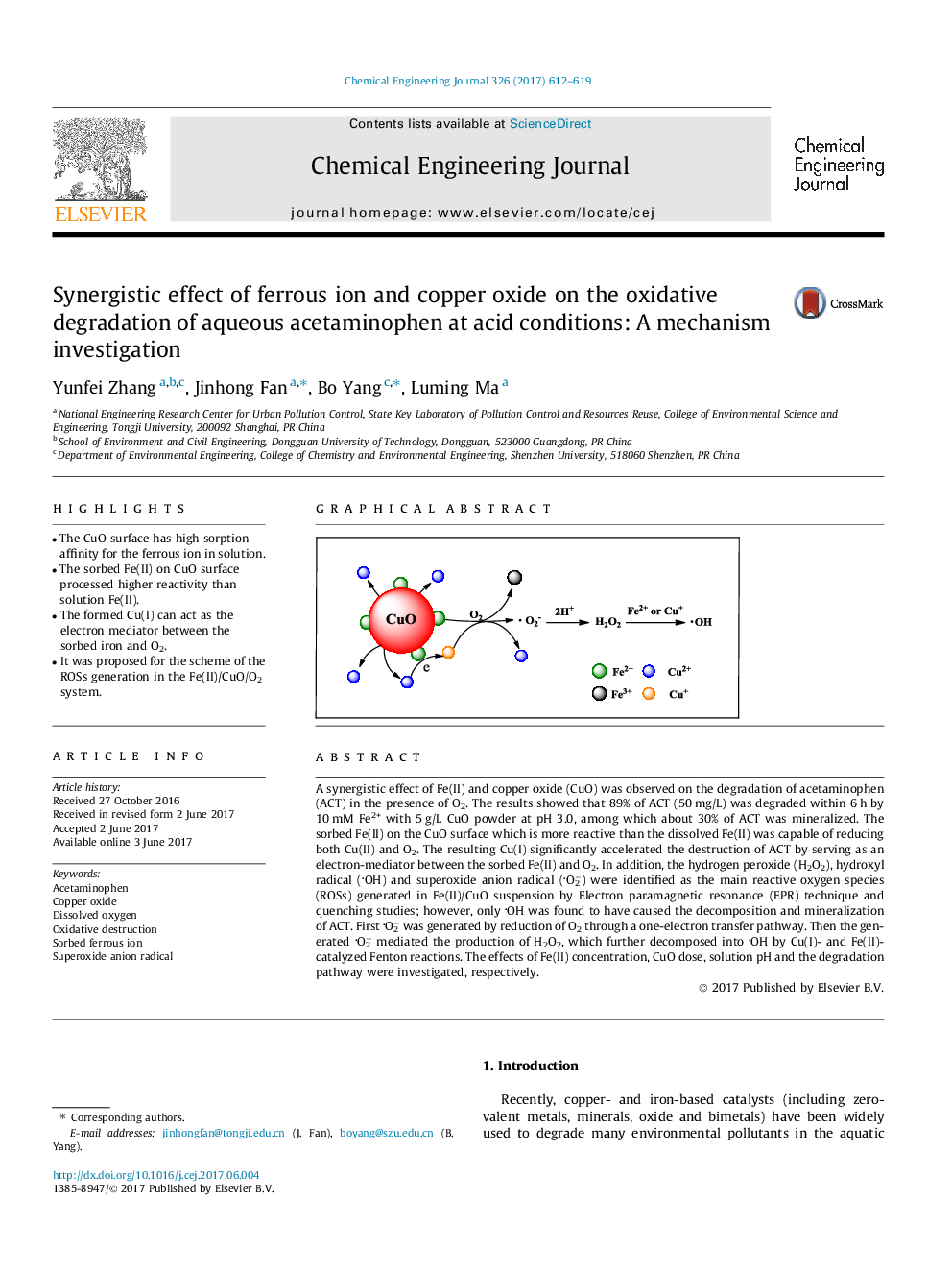
â¢The CuO surface has high sorption affinity for the ferrous ion in solution.â¢The sorbed Fe(II) on CuO surface processed higher reactivity than solution Fe(II).â¢The formed Cu(I) can act as the electron mediator between the sorbed iron and O2.â¢It was proposed for the scheme of the ROSs generation in the Fe(II)/CuO/O2 system.
A synergistic effect of Fe(II) and copper oxide (CuO) was observed on the degradation of acetaminophen (ACT) in the presence of O2. The results showed that 89% of ACT (50Â mg/L) was degraded within 6Â h by 10Â mM Fe2+ with 5Â g/L CuO powder at pH 3.0, among which about 30% of ACT was mineralized. The sorbed Fe(II) on the CuO surface which is more reactive than the dissolved Fe(II) was capable of reducing both Cu(II) and O2. The resulting Cu(I) significantly accelerated the destruction of ACT by serving as an electron-mediator between the sorbed Fe(II) and O2. In addition, the hydrogen peroxide (H2O2), hydroxyl radical (OH) and superoxide anion radical (O2â) were identified as the main reactive oxygen species (ROSs) generated in Fe(II)/CuO suspension by Electron paramagnetic resonance (EPR) technique and quenching studies; however, only OH was found to have caused the decomposition and mineralization of ACT. First O2â was generated by reduction of O2 through a one-electron transfer pathway. Then the generated O2â mediated the production of H2O2, which further decomposed into OH by Cu(I)- and Fe(II)-catalyzed Fenton reactions. The effects of Fe(II) concentration, CuO dose, solution pH and the degradation pathway were investigated, respectively.
Graphical abstractDownload high-res image (108KB)Download full-size image