| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 6465498 | Chemical Engineering Journal | 2017 | 9 Pages |
â¢First combination of MOFs with DFT calculations in adsorptive cobalt (II).â¢Metal-organic framework material was applied to cobalt (II) adsorption.â¢DFT calculations revealed the coordination modes between cobalt (II) and ligands.â¢The sorbent exhibits high sorption capacity for cobalt (II).â¢DFT calculations offers guidance on new MOF designs.
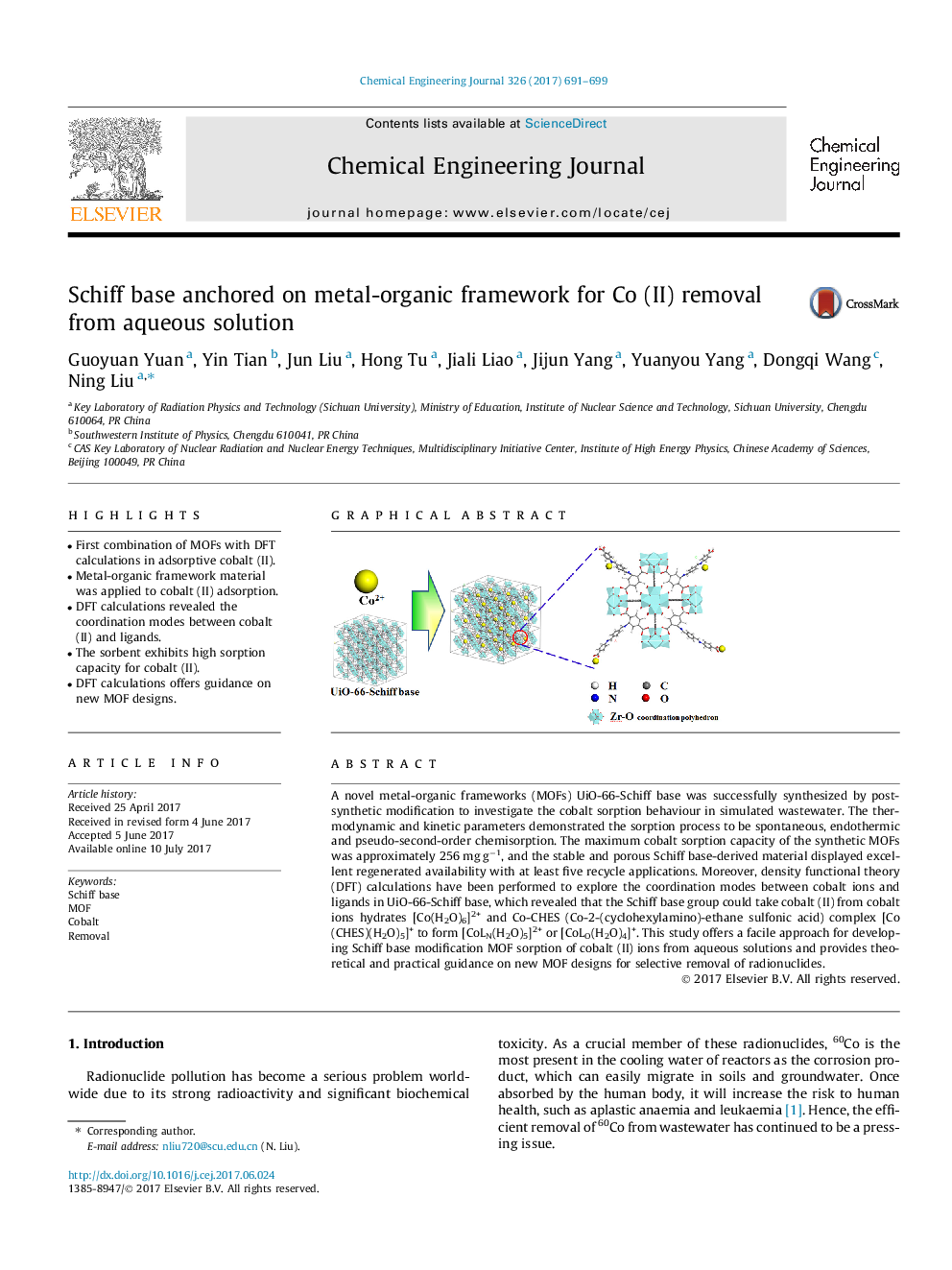
A novel metal-organic frameworks (MOFs) UiO-66-Schiff base was successfully synthesized by post-synthetic modification to investigate the cobalt sorption behaviour in simulated wastewater. The thermodynamic and kinetic parameters demonstrated the sorption process to be spontaneous, endothermic and pseudo-second-order chemisorption. The maximum cobalt sorption capacity of the synthetic MOFs was approximately 256 mg gâ1, and the stable and porous Schiff base-derived material displayed excellent regenerated availability with at least five recycle applications. Moreover, density functional theory (DFT) calculations have been performed to explore the coordination modes between cobalt ions and ligands in UiO-66-Schiff base, which revealed that the Schiff base group could take cobalt (II) from cobalt ions hydrates [Co(H2O)6]2+ and Co-CHES (Co-2-(cyclohexylamino)-ethane sulfonic acid) complex [Co(CHES)(H2O)5]+ to form [CoLN(H2O)5]2+ or [CoLO(H2O)4]+. This study offers a facile approach for developing Schiff base modification MOF sorption of cobalt (II) ions from aqueous solutions and provides theoretical and practical guidance on new MOF designs for selective removal of radionuclides.
Graphical abstractDownload high-res image (208KB)Download full-size image