| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 6465829 | Chemical Engineering Journal | 2017 | 9 Pages |
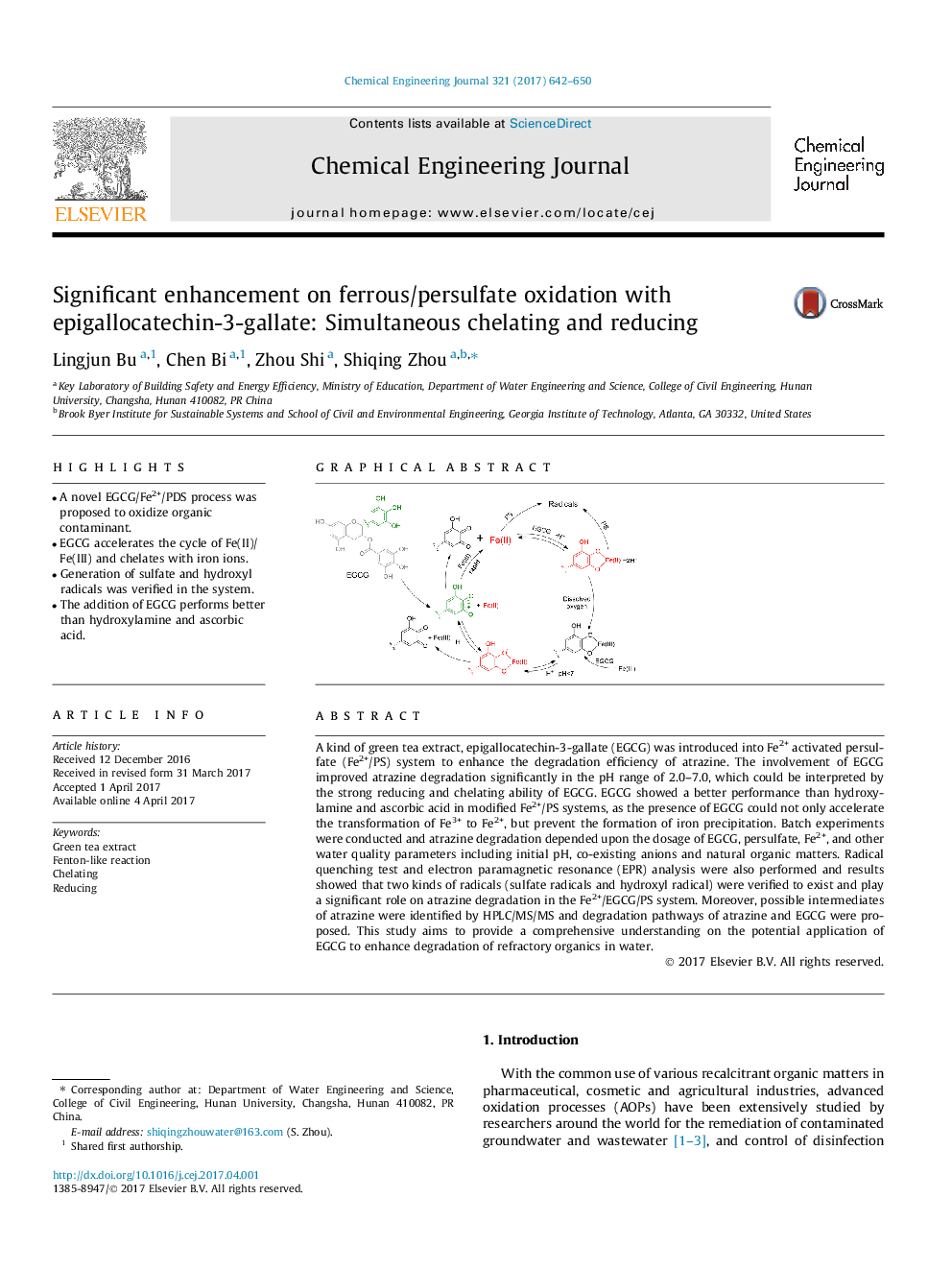
â¢A novel EGCG/Fe2+/PDS process was proposed to oxidize organic contaminant.â¢EGCG accelerates the cycle of Fe(II)/Fe(III) and chelates with iron ions.â¢Generation of sulfate and hydroxyl radicals was verified in the system.â¢The addition of EGCG performs better than hydroxylamine and ascorbic acid.
A kind of green tea extract, epigallocatechin-3-gallate (EGCG) was introduced into Fe2+ activated persulfate (Fe2+/PS) system to enhance the degradation efficiency of atrazine. The involvement of EGCG improved atrazine degradation significantly in the pH range of 2.0-7.0, which could be interpreted by the strong reducing and chelating ability of EGCG. EGCG showed a better performance than hydroxylamine and ascorbic acid in modified Fe2+/PS systems, as the presence of EGCG could not only accelerate the transformation of Fe3+ to Fe2+, but prevent the formation of iron precipitation. Batch experiments were conducted and atrazine degradation depended upon the dosage of EGCG, persulfate, Fe2+, and other water quality parameters including initial pH, co-existing anions and natural organic matters. Radical quenching test and electron paramagnetic resonance (EPR) analysis were also performed and results showed that two kinds of radicals (sulfate radicals and hydroxyl radical) were verified to exist and play a significant role on atrazine degradation in the Fe2+/EGCG/PS system. Moreover, possible intermediates of atrazine were identified by HPLC/MS/MS and degradation pathways of atrazine and EGCG were proposed. This study aims to provide a comprehensive understanding on the potential application of EGCG to enhance degradation of refractory organics in water.
Graphical abstractDownload high-res image (118KB)Download full-size image