| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 6466242 | Chemical Engineering Journal | 2017 | 11 Pages |
â¢CuOx/BiVO4 catalysts exhibit high activity for the degradation of BPA.â¢BPA degradation increases dramatically in the presence of bicarbonate.â¢Rate enhancement is due to the formation of large amounts of OH in solution.â¢OH radicals react with BPA through hydroxylation and scission of bonds.
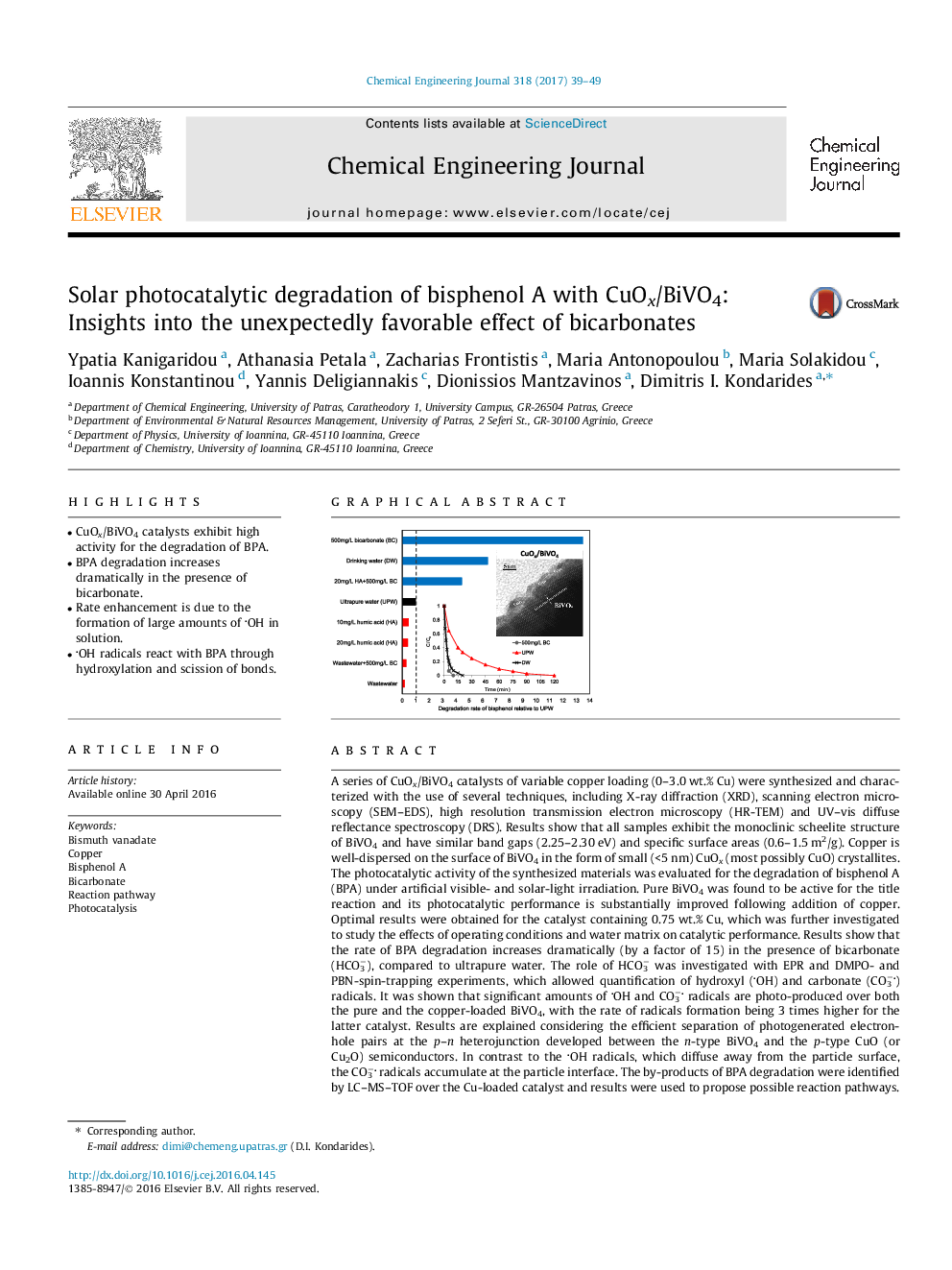
A series of CuOx/BiVO4 catalysts of variable copper loading (0-3.0Â wt.% Cu) were synthesized and characterized with the use of several techniques, including X-ray diffraction (XRD), scanning electron microscopy (SEM-EDS), high resolution transmission electron microscopy (HR-TEM) and UV-vis diffuse reflectance spectroscopy (DRS). Results show that all samples exhibit the monoclinic scheelite structure of BiVO4 and have similar band gaps (2.25-2.30Â eV) and specific surface areas (0.6-1.5Â m2/g). Copper is well-dispersed on the surface of BiVO4 in the form of small (<5Â nm) CuOx (most possibly CuO) crystallites. The photocatalytic activity of the synthesized materials was evaluated for the degradation of bisphenol A (BPA) under artificial visible- and solar-light irradiation. Pure BiVO4 was found to be active for the title reaction and its photocatalytic performance is substantially improved following addition of copper. Optimal results were obtained for the catalyst containing 0.75Â wt.% Cu, which was further investigated to study the effects of operating conditions and water matrix on catalytic performance. Results show that the rate of BPA degradation increases dramatically (by a factor of 15) in the presence of bicarbonate (HCO3â), compared to ultrapure water. The role of HCO3â was investigated with EPR and DMPO- and PBN-spin-trapping experiments, which allowed quantification of hydroxyl (OH) and carbonate (CO3â) radicals. It was shown that significant amounts of OH and CO3â radicals are photo-produced over both the pure and the copper-loaded BiVO4, with the rate of radicals formation being 3 times higher for the latter catalyst. Results are explained considering the efficient separation of photogenerated electron-hole pairs at the p-n heterojunction developed between the n-type BiVO4 and the p-type CuO (or Cu2O) semiconductors. In contrast to the OH radicals, which diffuse away from the particle surface, the CO3â radicals accumulate at the particle interface. The by-products of BPA degradation were identified by LC-MS-TOF over the Cu-loaded catalyst and results were used to propose possible reaction pathways. It is concluded that the exceptionally high photodegradation rate observed in the presence of HCO3â can be attributed to the formation of large amounts of OH radicals in the bulk solution, which react with BPA to yield reaction by-products through hydroxylation and scission of the bond between the isopropylidene carbon and the phenyl group.
Graphical abstractDownload high-res image (137KB)Download full-size image