| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 6466276 | Chemical Engineering Journal | 2017 | 8 Pages |
â¢Degradation of organic compounds by sulfate radical-based AOPs was evaluated.â¢An inhibition effect due to the presence of bromide was observed.â¢Degradation and mineralization of nitrobenzene was generally enhanced by halides.
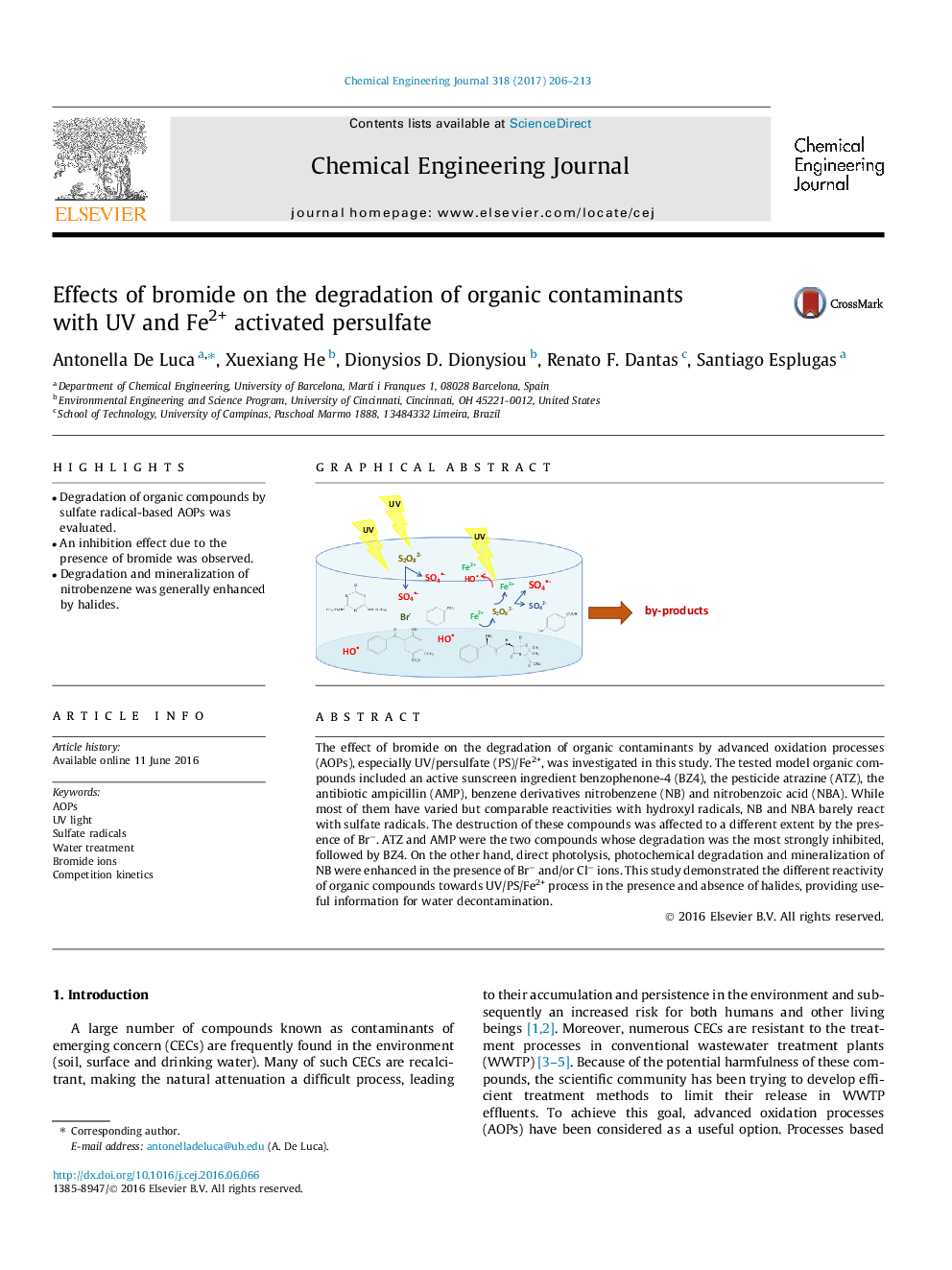
The effect of bromide on the degradation of organic contaminants by advanced oxidation processes (AOPs), especially UV/persulfate (PS)/Fe2+, was investigated in this study. The tested model organic compounds included an active sunscreen ingredient benzophenone-4 (BZ4), the pesticide atrazine (ATZ), the antibiotic ampicillin (AMP), benzene derivatives nitrobenzene (NB) and nitrobenzoic acid (NBA). While most of them have varied but comparable reactivities with hydroxyl radicals, NB and NBA barely react with sulfate radicals. The destruction of these compounds was affected to a different extent by the presence of Brâ. ATZ and AMP were the two compounds whose degradation was the most strongly inhibited, followed by BZ4. On the other hand, direct photolysis, photochemical degradation and mineralization of NB were enhanced in the presence of Brâ and/or Clâ ions. This study demonstrated the different reactivity of organic compounds towards UV/PS/Fe2+ process in the presence and absence of halides, providing useful information for water decontamination.
Graphical abstractDownload high-res image (105KB)Download full-size image