| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 6466347 | Chemical Engineering Journal | 2017 | 7 Pages |
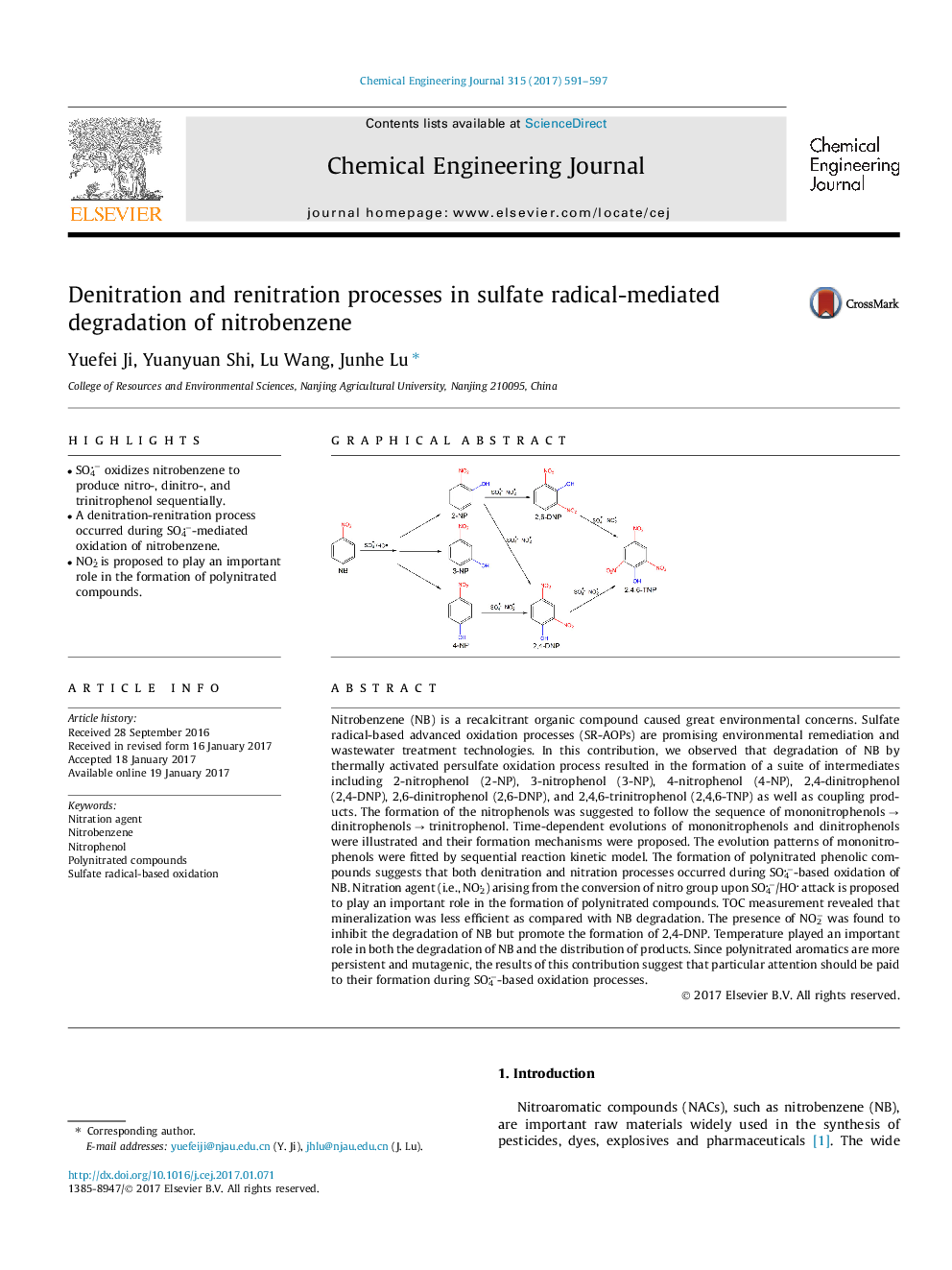
â¢SO4â oxidizes nitrobenzene to produce nitro-, dinitro-, and trinitrophenol sequentially.â¢A denitration-renitration process occurred during SO4â-mediated oxidation of nitrobenzene.â¢NO2 is proposed to play an important role in the formation of polynitrated compounds.
Nitrobenzene (NB) is a recalcitrant organic compound caused great environmental concerns. Sulfate radical-based advanced oxidation processes (SR-AOPs) are promising environmental remediation and wastewater treatment technologies. In this contribution, we observed that degradation of NB by thermally activated persulfate oxidation process resulted in the formation of a suite of intermediates including 2-nitrophenol (2-NP), 3-nitrophenol (3-NP), 4-nitrophenol (4-NP), 2,4-dinitrophenol (2,4-DNP), 2,6-dinitrophenol (2,6-DNP), and 2,4,6-trinitrophenol (2,4,6-TNP) as well as coupling products. The formation of the nitrophenols was suggested to follow the sequence of mononitrophenols â dinitrophenols â trinitrophenol. Time-dependent evolutions of mononitrophenols and dinitrophenols were illustrated and their formation mechanisms were proposed. The evolution patterns of mononitrophenols were fitted by sequential reaction kinetic model. The formation of polynitrated phenolic compounds suggests that both denitration and nitration processes occurred during SO4â-based oxidation of NB. Nitration agent (i.e., NO2) arising from the conversion of nitro group upon SO4â/HO attack is proposed to play an important role in the formation of polynitrated compounds. TOC measurement revealed that mineralization was less efficient as compared with NB degradation. The presence of NO2â was found to inhibit the degradation of NB but promote the formation of 2,4-DNP. Temperature played an important role in both the degradation of NB and the distribution of products. Since polynitrated aromatics are more persistent and mutagenic, the results of this contribution suggest that particular attention should be paid to their formation during SO4â-based oxidation processes.
Graphical abstractDownload high-res image (95KB)Download full-size image