| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 6466611 | Chemical Engineering Journal | 2017 | 8 Pages |
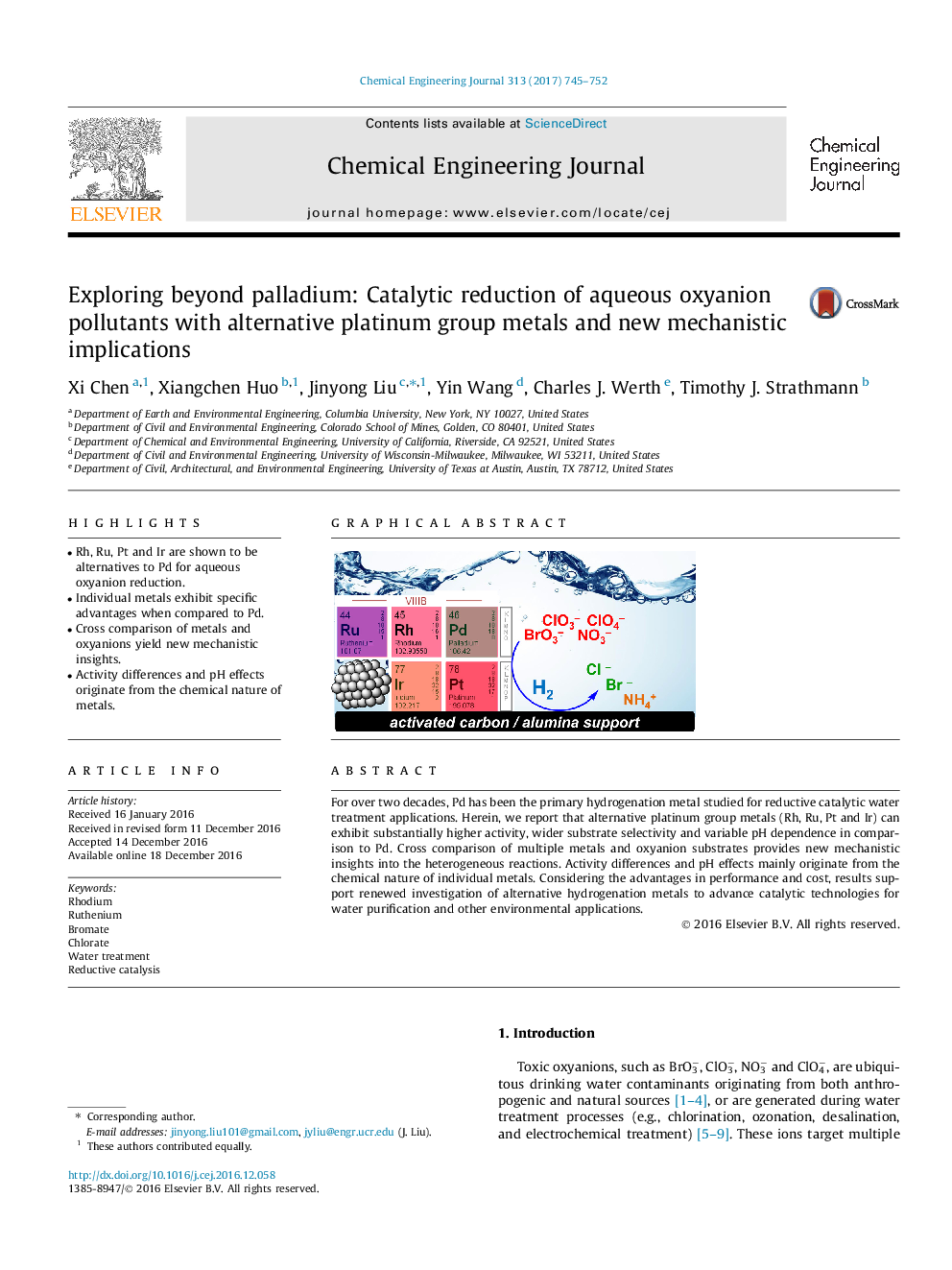
•Rh, Ru, Pt and Ir are shown to be alternatives to Pd for aqueous oxyanion reduction.•Individual metals exhibit specific advantages when compared to Pd.•Cross comparison of metals and oxyanions yield new mechanistic insights.•Activity differences and pH effects originate from the chemical nature of metals.
For over two decades, Pd has been the primary hydrogenation metal studied for reductive catalytic water treatment applications. Herein, we report that alternative platinum group metals (Rh, Ru, Pt and Ir) can exhibit substantially higher activity, wider substrate selectivity and variable pH dependence in comparison to Pd. Cross comparison of multiple metals and oxyanion substrates provides new mechanistic insights into the heterogeneous reactions. Activity differences and pH effects mainly originate from the chemical nature of individual metals. Considering the advantages in performance and cost, results support renewed investigation of alternative hydrogenation metals to advance catalytic technologies for water purification and other environmental applications.
Graphical abstractFigure optionsDownload full-size imageDownload high-quality image (126 K)Download as PowerPoint slide