| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 6466714 | Chemical Engineering Journal | 2017 | 10 Pages |
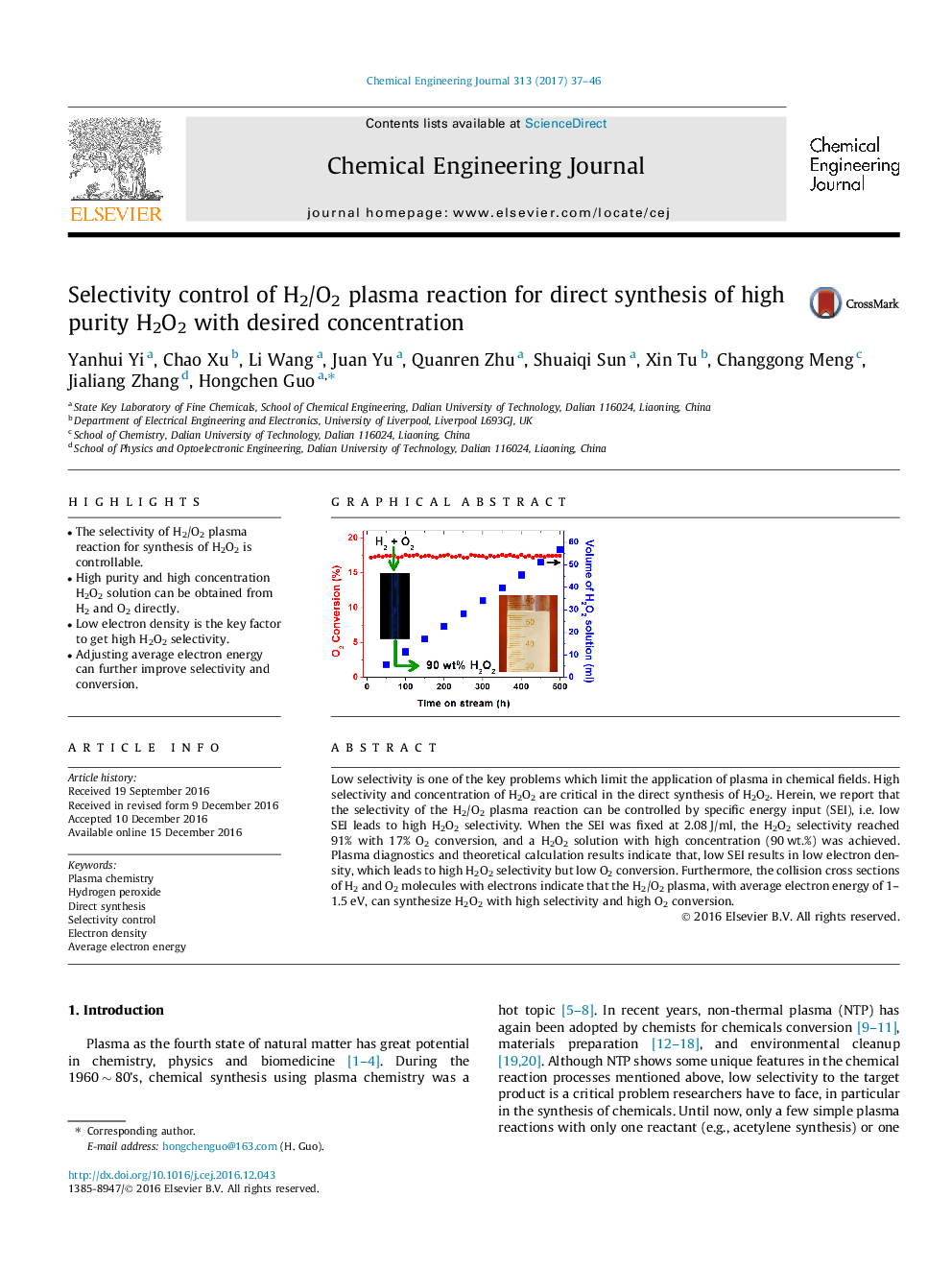
â¢The selectivity of H2/O2 plasma reaction for synthesis of H2O2 is controllable.â¢High purity and high concentration H2O2 solution can be obtained from H2 and O2 directly.â¢Low electron density is the key factor to get high H2O2 selectivity.â¢Adjusting average electron energy can further improve selectivity and conversion.
Low selectivity is one of the key problems which limit the application of plasma in chemical fields. High selectivity and concentration of H2O2 are critical in the direct synthesis of H2O2. Herein, we report that the selectivity of the H2/O2 plasma reaction can be controlled by specific energy input (SEI), i.e. low SEI leads to high H2O2 selectivity. When the SEI was fixed at 2.08Â J/ml, the H2O2 selectivity reached 91% with 17% O2 conversion, and a H2O2 solution with high concentration (90Â wt.%) was achieved. Plasma diagnostics and theoretical calculation results indicate that, low SEI results in low electron density, which leads to high H2O2 selectivity but low O2 conversion. Furthermore, the collision cross sections of H2 and O2 molecules with electrons indicate that the H2/O2 plasma, with average electron energy of 1-1.5Â eV, can synthesize H2O2 with high selectivity and high O2 conversion.
Graphical abstractDownload high-res image (92KB)Download full-size image