| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 2038995 | Cell Reports | 2016 | 13 Pages |
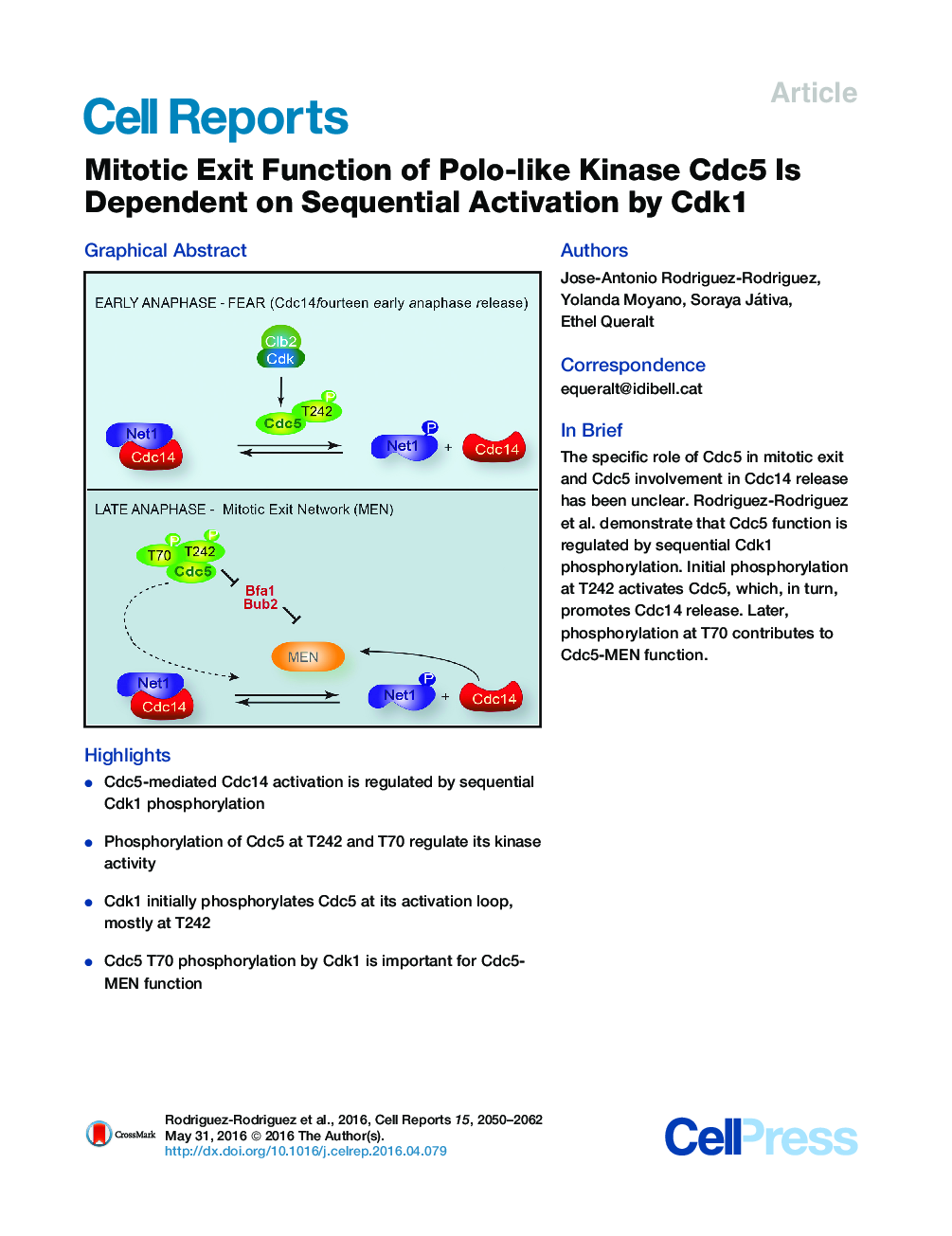
•Cdc5-mediated Cdc14 activation is regulated by sequential Cdk1 phosphorylation•Phosphorylation of Cdc5 at T242 and T70 regulate its kinase activity•Cdk1 initially phosphorylates Cdc5 at its activation loop, mostly at T242•Cdc5 T70 phosphorylation by Cdk1 is important for Cdc5-MEN function
SummaryTo complete mitosis, Saccharomyces cerevisiae needs to activate the mitotic phosphatase Cdc14. Two pathways contribute to Cdc14 regulation: FEAR (Cdc14 early anaphase release) and MEN (mitotic exit network). Cdc5 polo-like kinase was found to be an important mitotic exit component. However, its specific role in mitotic exit regulation and its involvement in Cdc14 release remain unclear. Here, we provide insight into the mechanism by which Cdc5 contributes to the timely release of Cdc14. Our genetic and biochemical data indicate that Cdc5 acts in parallel with MEN during anaphase. This MEN-independent Cdc5 function requires active separase and activation by Cdk1-dependent phosphorylation. Cdk1 first phosphorylates Cdc5 to activate it in early anaphase, and then, in late anaphase, further phosphorylation of Cdc5 by Cdk1 is needed to promote its MEN-related functions.
Graphical AbstractFigure optionsDownload full-size imageDownload as PowerPoint slide