| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 2039131 | Cell Reports | 2016 | 9 Pages |
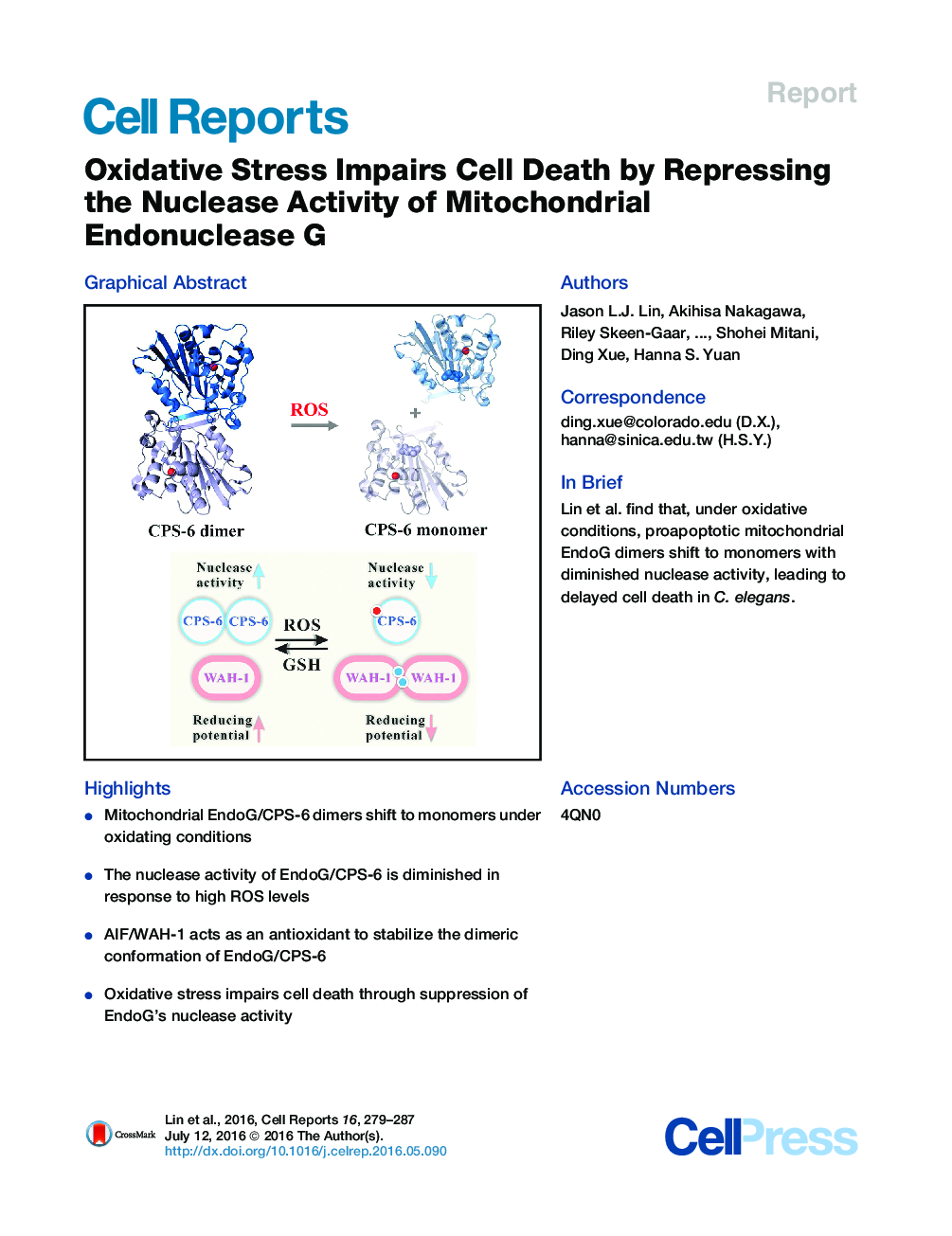
•Mitochondrial EndoG/CPS-6 dimers shift to monomers under oxidating conditions•The nuclease activity of EndoG/CPS-6 is diminished in response to high ROS levels•AIF/WAH-1 acts as an antioxidant to stabilize the dimeric conformation of EndoG/CPS-6•Oxidative stress impairs cell death through suppression of EndoG’s nuclease activity
SummaryEndonuclease G (EndoG) is a mitochondrial protein that is released from mitochondria and relocated into the nucleus to promote chromosomal DNA fragmentation during apoptosis. Here, we show that oxidative stress causes cell-death defects in C. elegans through an EndoG-mediated cell-death pathway. In response to high reactive oxygen species (ROS) levels, homodimeric CPS-6—the C. elegans homolog of EndoG—is dissociated into monomers with diminished nuclease activity. Conversely, the nuclease activity of CPS-6 is enhanced, and its dimeric structure is stabilized by its interaction with the worm AIF homolog, WAH-1, which shifts to disulfide cross-linked dimers under high ROS levels. CPS-6 thus acts as a ROS sensor to regulate the life and death of cells. Modulation of the EndoG dimer conformation could present an avenue for prevention and treatment of diseases resulting from oxidative stress.
Graphical AbstractFigure optionsDownload full-size imageDownload as PowerPoint slide