| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 2039357 | Cell Reports | 2016 | 14 Pages |
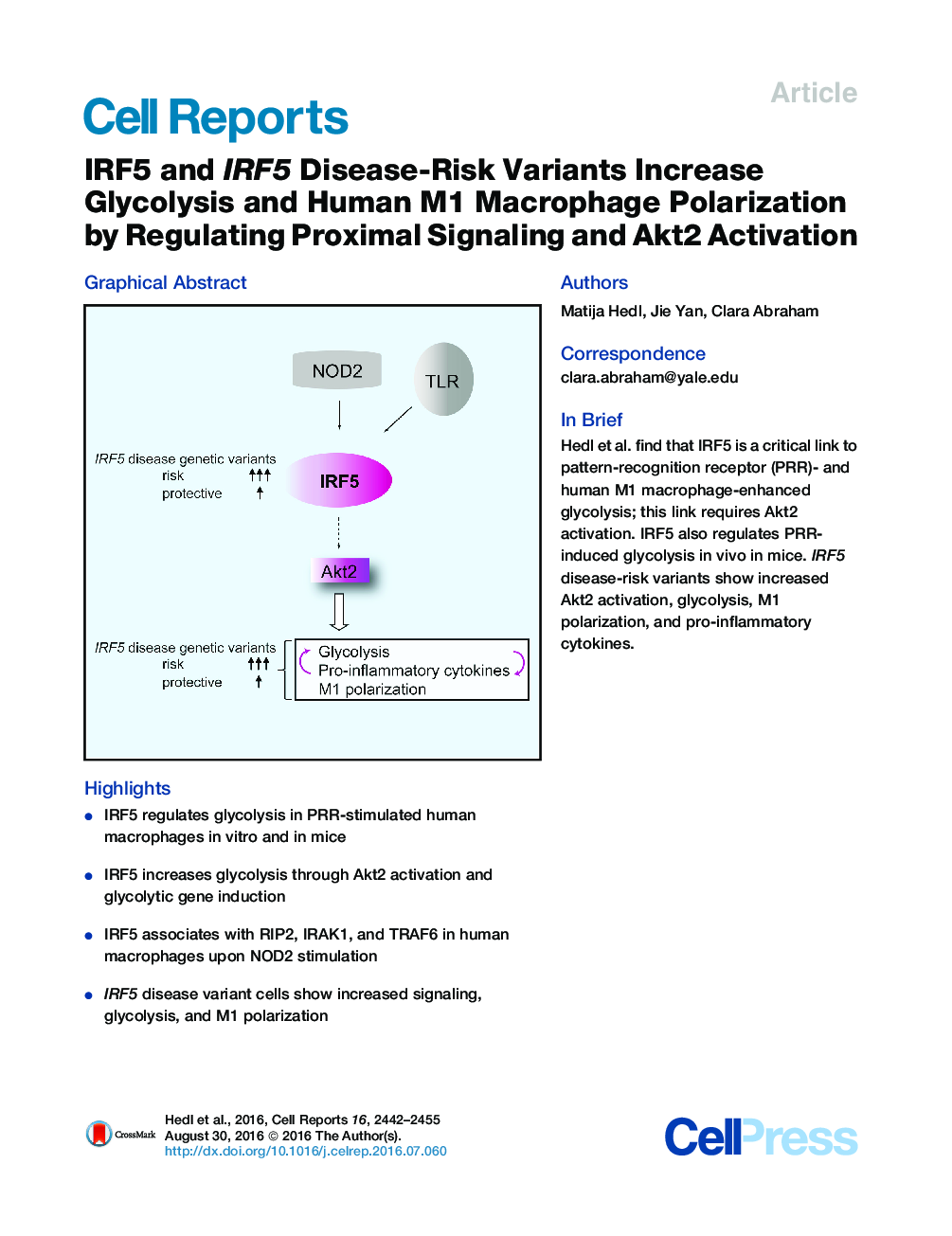
•IRF5 regulates glycolysis in PRR-stimulated human macrophages in vitro and in mice•IRF5 increases glycolysis through Akt2 activation and glycolytic gene induction•IRF5 associates with RIP2, IRAK1, and TRAF6 in human macrophages upon NOD2 stimulation•IRF5 disease variant cells show increased signaling, glycolysis, and M1 polarization
SummaryInterferon regulatory factor 5 (IRF5) regulates inflammatory M1 macrophage polarization, and disease-associated IRF5 genetic variants regulate pattern-recognition-receptor (PRR)-induced cytokines. PRR-stimulated macrophages and M1 macrophages exhibit enhanced glycolysis, a central mediator of inflammation. We find that IRF5 is needed for PRR-enhanced glycolysis in human macrophages and in mice in vivo. Upon stimulation of the PRR nucleotide binding oligomerization domain containing 2 (NOD2) in human macrophages, IRF5 binds RIP2, IRAK1, and TRAF6. IRF5, in turn, is required for optimal Akt2 activation, which increases expression of glycolytic pathway genes and HIF1A as well as pro-inflammatory cytokines and M1 polarization. Furthermore, pro-inflammatory cytokines and glycolytic pathways co-regulate each other. Rs2004640/rs2280714 TT/TT IRF5 disease-risk-carrier cells demonstrate increased IRF5 expression and increased PRR-induced Akt2 activation, glycolysis, pro-inflammatory cytokines, and M1 polarization relative to GG/CC carrier macrophages. Our findings identify that IRF5 disease-associated polymorphisms regulate diverse immunological and metabolic outcomes and provide further insight into mechanisms contributing to the increasingly recognized important role for glycolysis in inflammation.
Graphical AbstractFigure optionsDownload full-size imageDownload as PowerPoint slide