| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 2039780 | Cell Reports | 2016 | 9 Pages |
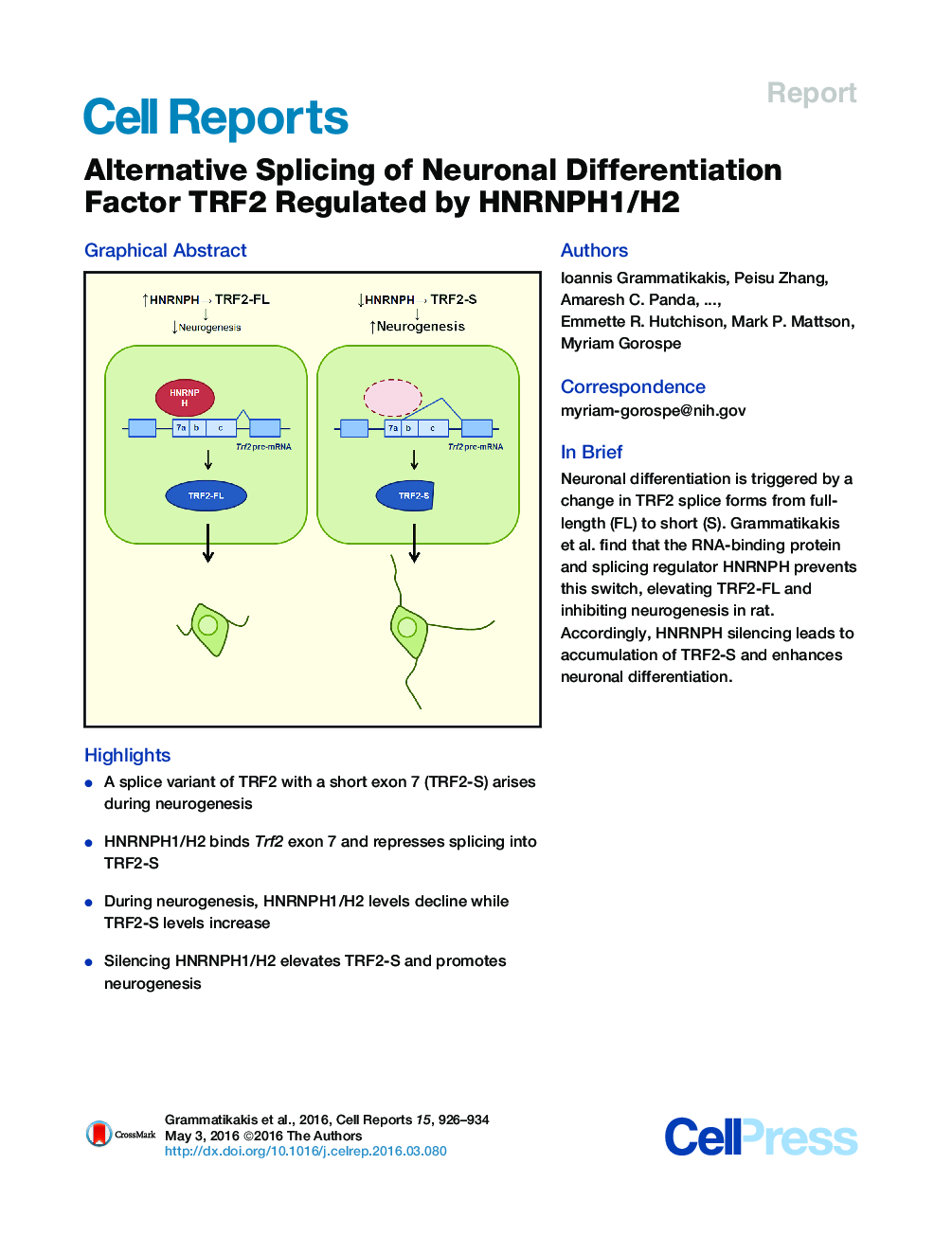
•A splice variant of TRF2 with a short exon 7 (TRF2-S) arises during neurogenesis•HNRNPH1/H2 binds Trf2 exon 7 and represses splicing into TRF2-S•During neurogenesis, HNRNPH1/H2 levels decline while TRF2-S levels increase•Silencing HNRNPH1/H2 elevates TRF2-S and promotes neurogenesis
SummaryDuring neuronal differentiation, use of an alternative splice site on the rat telomere repeat-binding factor 2 (TRF2) mRNA generates a short TRF2 protein isoform (TRF2-S) capable of derepressing neuronal genes. However, the RNA-binding proteins (RBPs) controlling this splicing event are unknown. Here, using affinity pull-down analysis, we identified heterogeneous nuclear ribonucleoproteins H1 and H2(HNRNPH) as RBPs specifically capable of interacting with the spliced RNA segment (exon 7) of Trf2 pre-mRNA. HNRNPH proteins prevent the production of the short isoform of Trf2 mRNA, as HNRNPH silencing selectively elevates TRF2-S levels. Accordingly, HNRNPH levels decline while TRF2-S levels increase during neuronal differentiation. In addition, CRISPR/Cas9-mediated deletion of hnRNPH2 selectively accelerates the NGF-triggered differentiation of rat pheochromocytoma cells into neurons. In sum, HNRNPH is a splicing regulator of Trf2 pre-mRNA that prevents the expression of TRF2-S, a factor implicated in neuronal differentiation.
Graphical AbstractFigure optionsDownload full-size imageDownload as PowerPoint slide