| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 2040006 | Cell Reports | 2016 | 16 Pages |
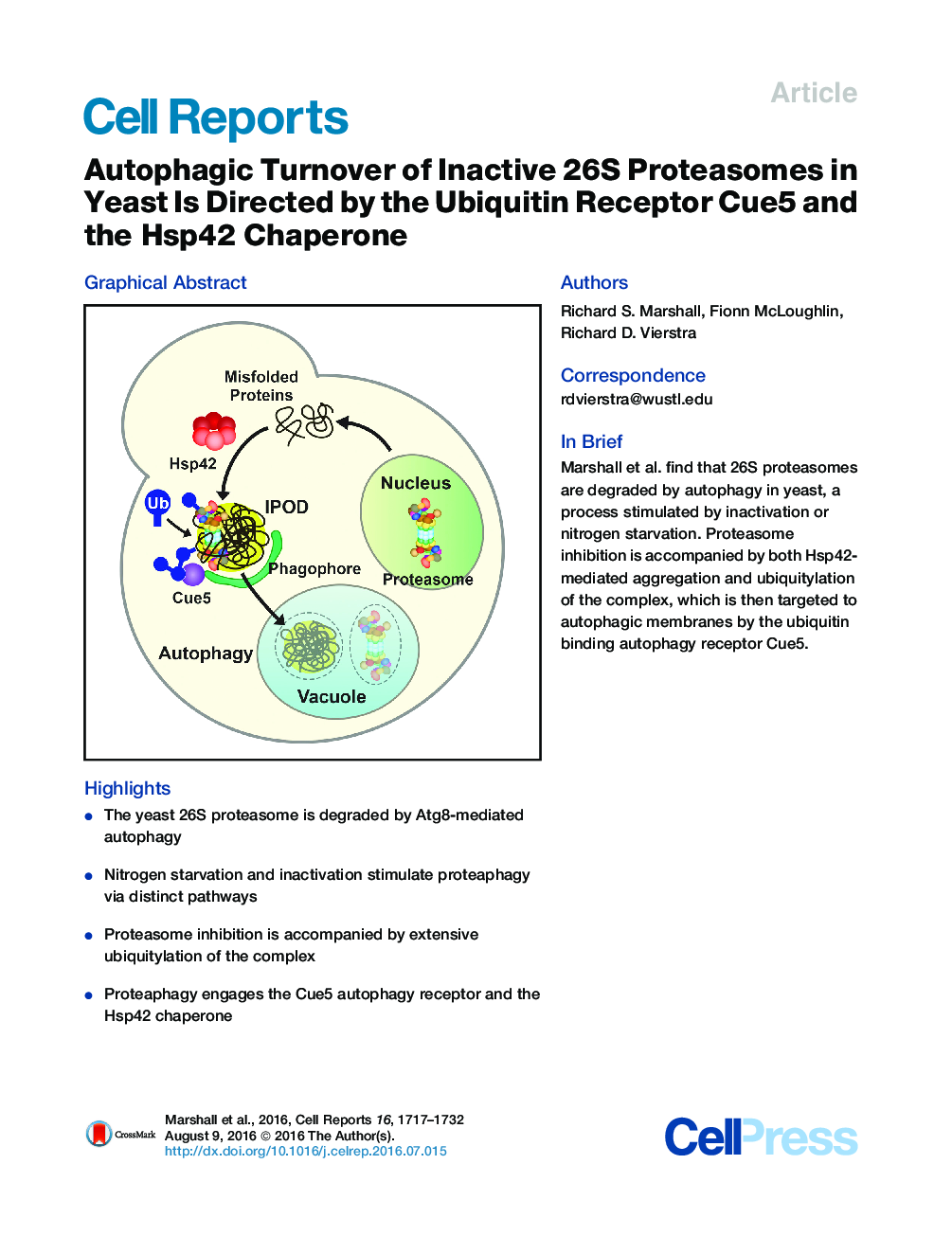
•The yeast 26S proteasome is degraded by Atg8-mediated autophagy•Nitrogen starvation and inactivation stimulate proteaphagy via distinct pathways•Proteasome inhibition is accompanied by extensive ubiquitylation of the complex•Proteaphagy engages the Cue5 autophagy receptor and the Hsp42 chaperone
SummaryThe autophagic clearance of 26S proteasomes (proteaphagy) is an important homeostatic mechanism within the ubiquitin system that modulates proteolytic capacity and eliminates damaged particles. Here, we define two proteaphagy routes in yeast that respond to either nitrogen starvation or particle inactivation. Whereas the core autophagic machineries required for Atg8 lipidation and vesiculation are essential for both routes, the upstream Atg1 kinase participates only in starvation-induced proteaphagy. Following inactivation, 26S proteasomes become extensively modified with ubiquitin. Although prior studies with Arabidopsis implicated RPN10 in tethering ubiquitylated proteasomes to ATG8 lining the autophagic membranes, yeast proteaphagy employs the evolutionarily distinct receptor Cue5, which simultaneously binds ubiquitin and Atg8. Proteaphagy of inactivated proteasomes also requires the oligomeric Hsp42 chaperone, suggesting that ubiquitylated proteasomes are directed by Hsp42 to insoluble protein deposit (IPOD)-type structures before encapsulation. Together, Cue5 and Hsp42 provide a quality control checkpoint in yeast directed at recycling dysfunctional 26S proteasomes.
Graphical AbstractFigure optionsDownload full-size imageDownload as PowerPoint slide