| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 2041132 | Cell Reports | 2015 | 8 Pages |
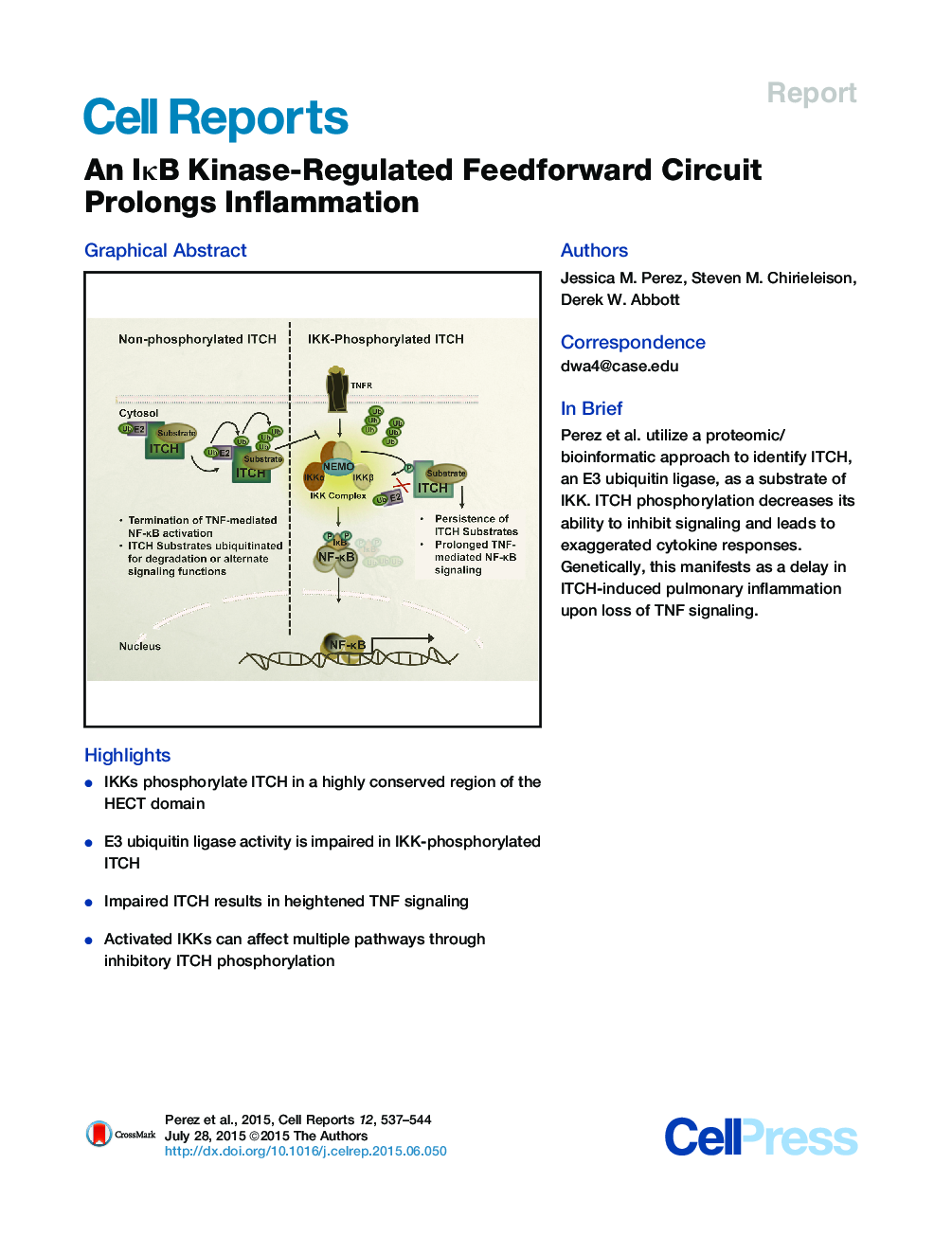
•IKKs phosphorylate ITCH in a highly conserved region of the HECT domain•E3 ubiquitin ligase activity is impaired in IKK-phosphorylated ITCH•Impaired ITCH results in heightened TNF signaling•Activated IKKs can affect multiple pathways through inhibitory ITCH phosphorylation
SummaryLoss of NF-κB signaling causes immunodeficiency, whereas inhibition of NF-κB can be efficacious in treating chronic inflammatory disease. Inflammatory NF-κB signaling must therefore be tightly regulated, and although many mechanisms to downregulate NF-κB have been elucidated, there have only been limited studies demonstrating positive feedforward regulation of NF-κB signaling. In this work, we use a bioinformatic and proteomic approach to discover that the IKK family of proteins can phosphorylate the E3 ubiquitin ligase ITCH, a critical downregulator of TNF-mediated NF-κB activation. Phosphorylation of ITCH by IKKs leads to impaired ITCH E3 ubiquitin ligase activity and prolongs NF-κB signaling and pro-inflammatory cytokine release. Since genetic loss of ITCH mirrors IKK-induced ITCH phosphorylation, we further show that the ITCH−/− mouse’s spontaneous lung inflammation and subsequent death can be delayed when TNF signaling is genetically deleted. This work identifies a new positive feedforward regulation of NF-κB activation that drives inflammatory disease.
Graphical AbstractFigure optionsDownload full-size imageDownload as PowerPoint slide