| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 2041621 | Cell Reports | 2016 | 13 Pages |
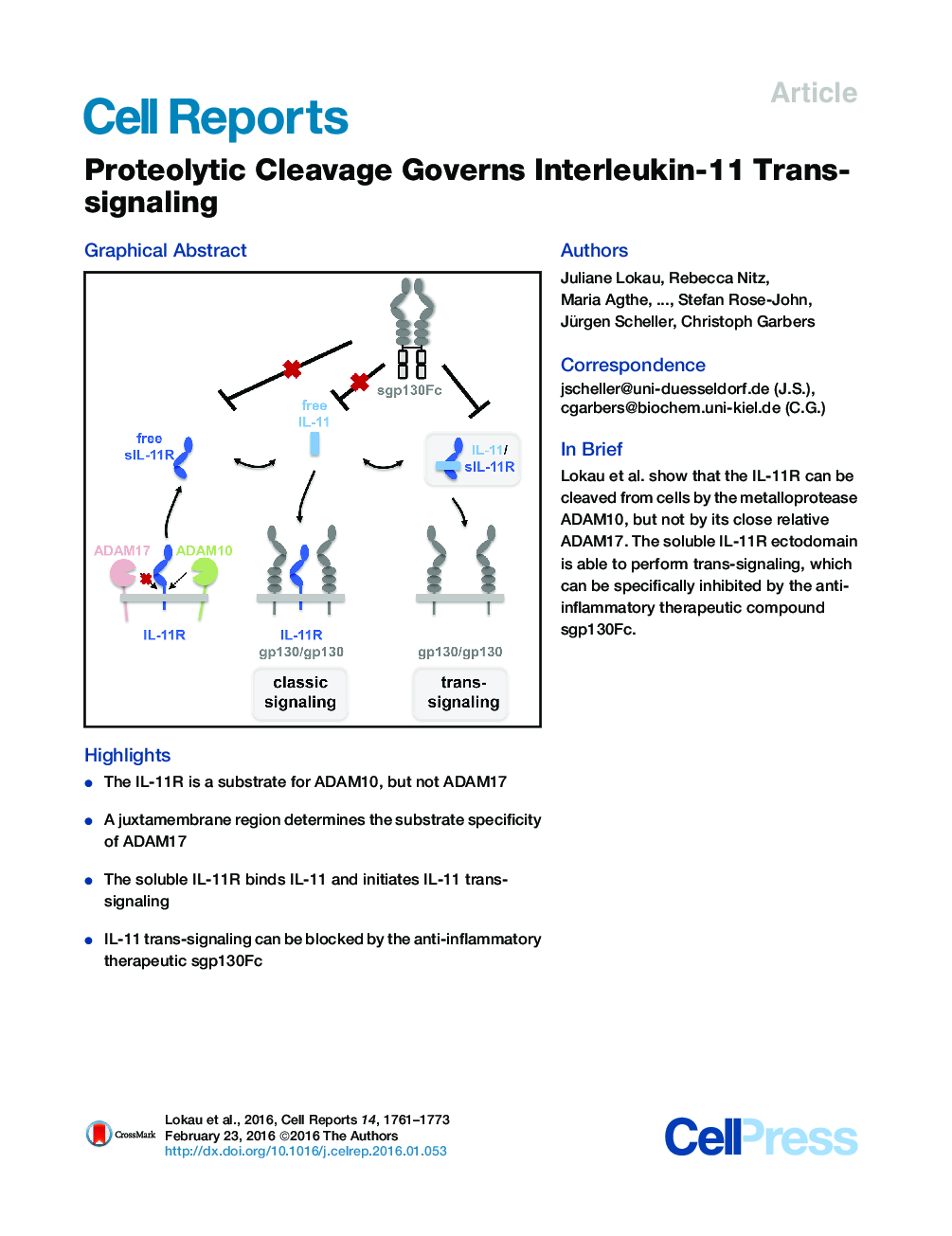
•The IL-11R is a substrate for ADAM10, but not ADAM17•A juxtamembrane region determines the substrate specificity of ADAM17•The soluble IL-11R binds IL-11 and initiates IL-11 trans-signaling•IL-11 trans-signaling can be blocked by the anti-inflammatory therapeutic sgp130Fc
SummaryInterleukin (IL)-11 has been shown to be a crucial factor for intestinal tumorigenesis, lung carcinomas, and asthma. IL-11 is thought to exclusively mediate its biological functions through cell-type-specific expression of the membrane-bound IL-11 receptor (IL-11R). Here, we show that the metalloprotease ADAM10, but not ADAM17, can release the IL-11R ectodomain. Chimeric proteins of the IL-11R and the IL-6 receptor (IL-6R) revealed that a small juxtamembrane portion is responsible for this substrate specificity of ADAM17. Furthermore, we show that the serine proteases neutrophil elastase and proteinase 3 can also cleave the IL-11R. The resulting soluble IL-11R (sIL-11R) is biologically active and binds IL-11 to activate cells. This IL-11 trans-signaling pathway can be inhibited specifically by the anti-inflammatory therapeutic compound sgp130Fc. In conclusion, proteolysis of the IL-11R represents a molecular switch that controls the IL-11 trans-signaling pathway and widens the number of cells that can be activated by IL-11.
Graphical AbstractFigure optionsDownload full-size imageDownload as PowerPoint slide