| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 2041853 | Cell Reports | 2015 | 11 Pages |
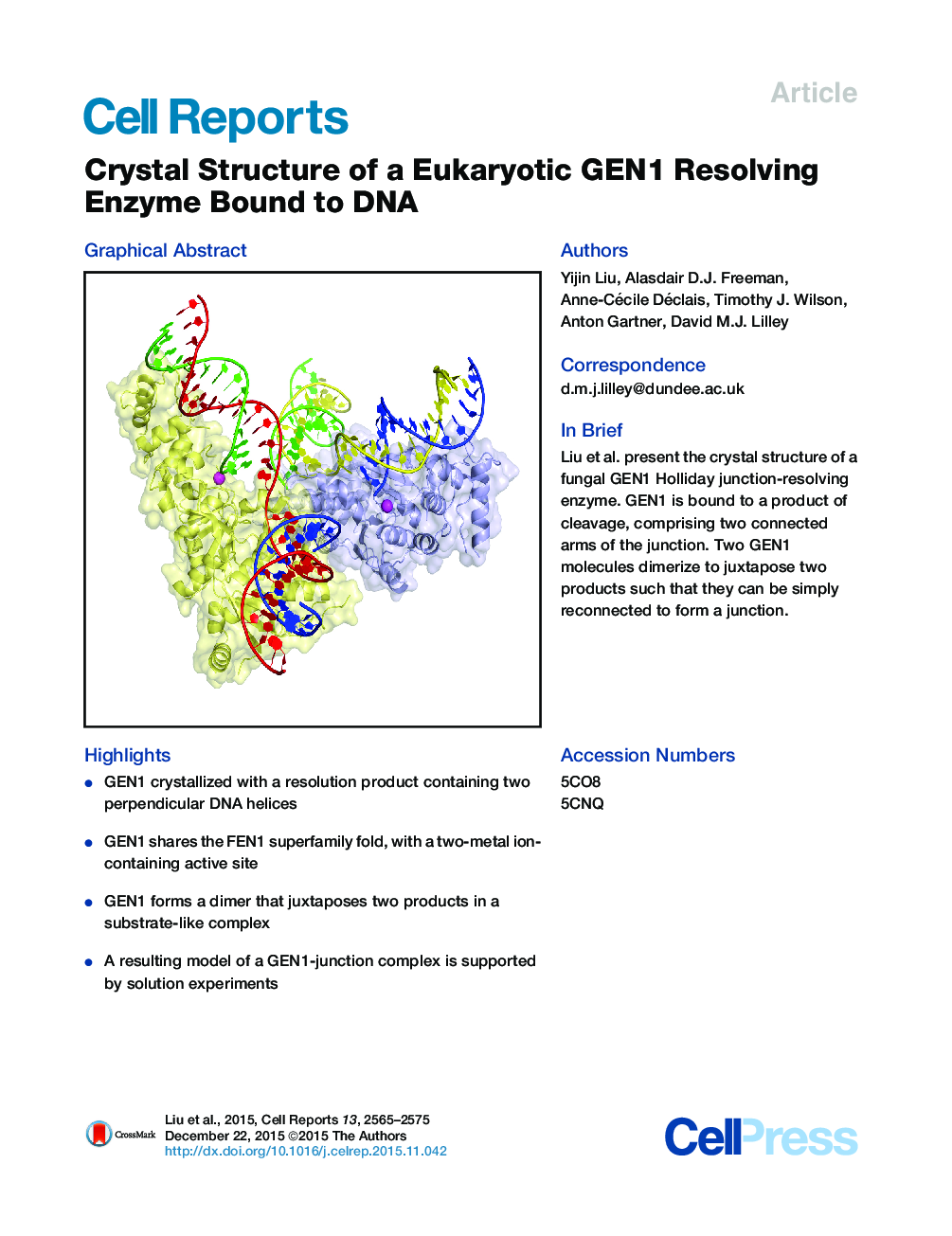
•GEN1 crystallized with a resolution product containing two perpendicular DNA helices•GEN1 shares the FEN1 superfamily fold, with a two-metal ion-containing active site•GEN1 forms a dimer that juxtaposes two products in a substrate-like complex•A resulting model of a GEN1-junction complex is supported by solution experiments
SummaryWe present the crystal structure of the junction-resolving enzyme GEN1 bound to DNA at 2.5 Å resolution. The structure of the GEN1 protein reveals it to have an elaborated FEN-XPG family fold that is modified for its role in four-way junction resolution. The functional unit in the crystal is a monomer of active GEN1 bound to the product of resolution cleavage, with an extensive DNA binding interface for both helical arms. Within the crystal lattice, a GEN1 dimer interface juxtaposes two products, whereby they can be reconnected into a four-way junction, the structure of which agrees with that determined in solution. The reconnection requires some opening of the DNA structure at the center, in agreement with permanganate probing and 2-aminopurine fluorescence. The structure shows that a relaxation of the DNA structure accompanies cleavage, suggesting how second-strand cleavage is accelerated to ensure productive resolution of the junction.
Graphical AbstractFigure optionsDownload full-size imageDownload as PowerPoint slide