| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 2042150 | Cell Reports | 2014 | 15 Pages |
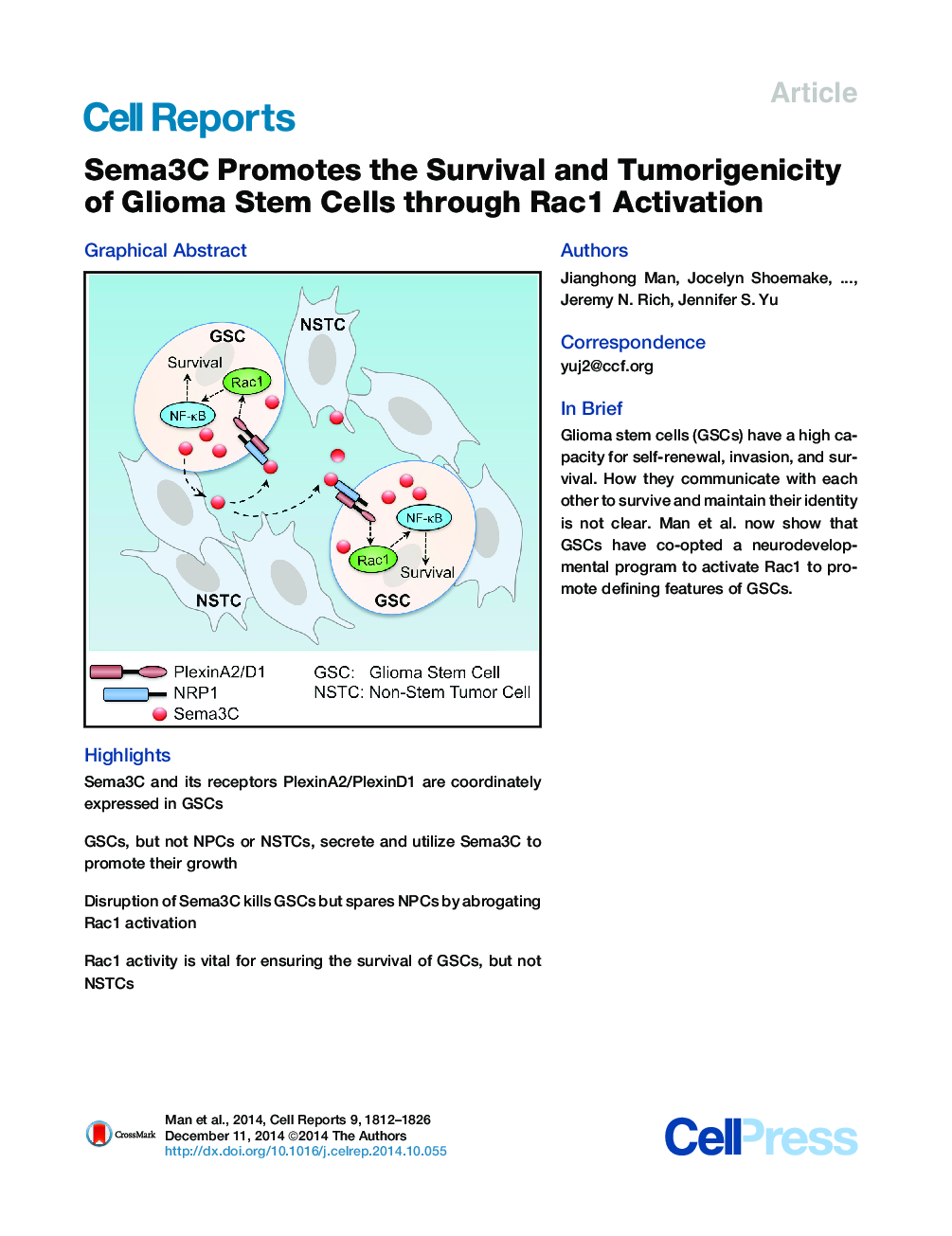
•Sema3C and its receptors PlexinA2/PlexinD1 are coordinately expressed in GSCs•GSCs, but not NPCs or NSTCs, secrete and utilize Sema3C to promote their growth•Disruption of Sema3C kills GSCs but spares NPCs by abrogating Rac1 activation•Rac1 activity is vital for ensuring the survival of GSCs, but not NSTCs
SummaryDifferent cancer cell compartments often communicate through soluble factors to facilitate tumor growth. Glioma stem cells (GSCs) are a subset of tumor cells that resist standard therapy to contribute to disease progression. How GSCs employ a distinct secretory program to communicate with and nurture each other over the nonstem tumor cell (NSTC) population is not well defined. Here, we show that GSCs preferentially secrete Sema3C and coordinately express PlexinA2/D1 receptors to activate Rac1/nuclear factor (NF)-κB signaling in an autocrine/paracrine loop to promote their own survival. Importantly, Sema3C is not expressed in neural progenitor cells (NPCs) or NSTCs. Disruption of Sema3C induced apoptosis of GSCs, but not NPCs or NSTCs, and suppressed tumor growth in orthotopic models of glioblastoma. Introduction of activated Rac1 rescued the Sema3C knockdown phenotype in vivo. Our study supports the targeting of Sema3C to break this GSC-specific autocrine/paracrine loop in order to improve glioblastoma treatment, potentially with a high therapeutic index.
Graphical AbstractFigure optionsDownload full-size imageDownload as PowerPoint slide