| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 2042151 | Cell Reports | 2014 | 14 Pages |
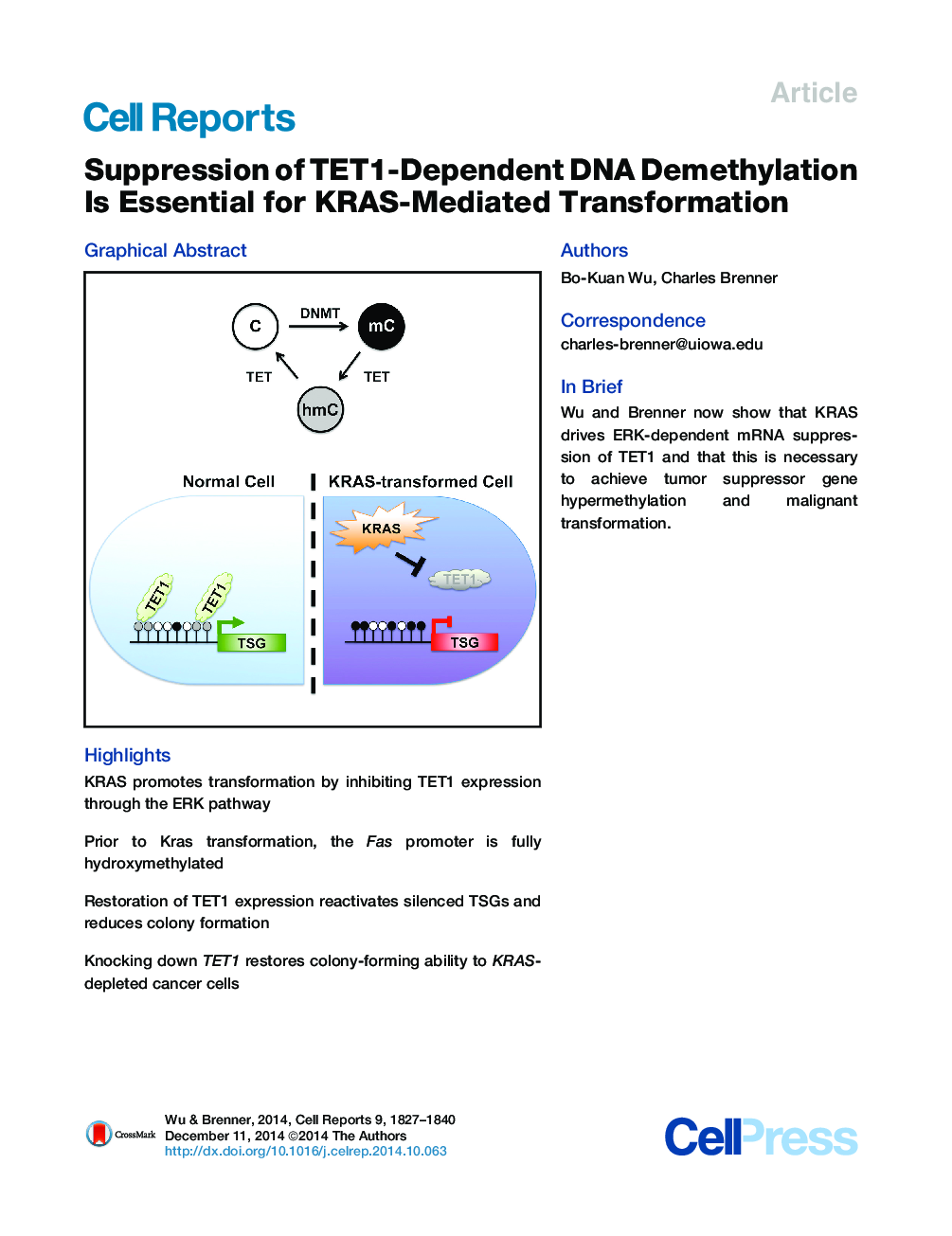
•KRAS promotes transformation by inhibiting TET1 expression through the ERK pathway•Prior to Kras transformation, the Fas promoter is fully hydroxymethylated•Restoration of TET1 expression reactivates silenced TSGs and reduces colony formation•Knocking down TET1 restores colony-forming ability to KRAS-depleted cancer cells
SummaryHypermethylation-mediated tumor suppressor gene (TSG) silencing is a central epigenetic alteration in RAS-dependent tumorigenesis. Ten-eleven translocation (TET) enzymes can depress DNA methylation by hydroxylation of 5-methylcytosine (5mC) bases to 5-hydroxymethylcytosine (5hmC). Here, we report that suppression of TET1 is required for KRAS-induced DNA hypermethylation and cellular transformation. In distinct nonmalignant cell lines, oncogenic KRAS promotes transformation by inhibiting TET1 expression via the ERK-signaling pathway. This reduces chromatin occupancy of TET1 at TSG promoters, lowers levels of 5hmC, and increases levels of 5mC and 5mC-dependent transcriptional silencing. Restoration of TET1 expression by ERK pathway inhibition or ectopic TET1 reintroduction in KRAS-transformed cells reactivates TSGs and inhibits colony formation. KRAS knockdown increases TET1 expression and diminishes colony-forming ability, whereas KRAS/TET1 double knockdown bypasses the KRAS dependence of KRAS-addicted cancer cells. Thus, suppression of TET1-dependent DNA demethylation is critical for KRAS-mediated transformation.
Graphical AbstractFigure optionsDownload full-size imageDownload as PowerPoint slide