| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 2028412 | 1070415 | 2012 | 10 صفحه PDF | دانلود رایگان |
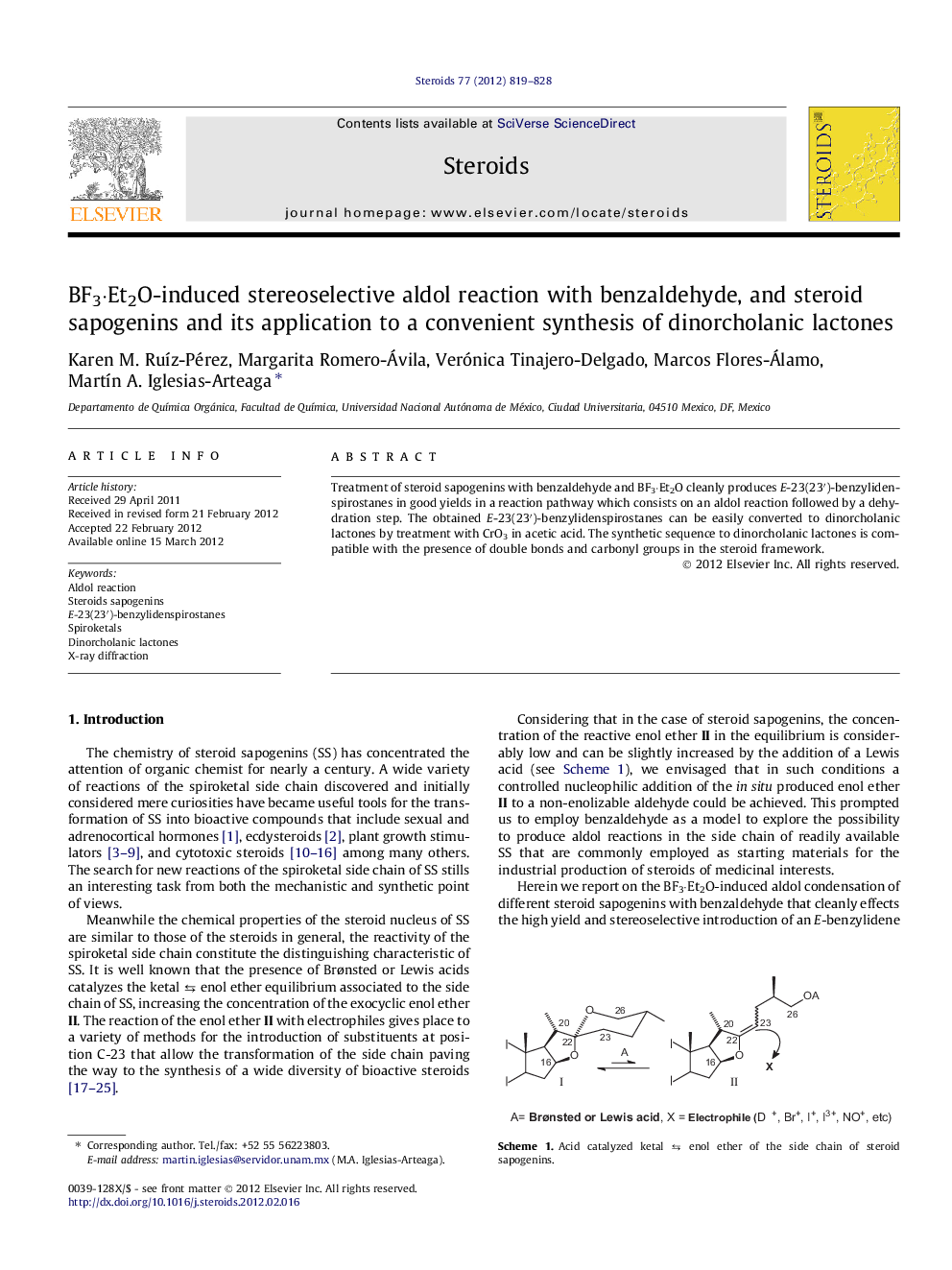
Treatment of steroid sapogenins with benzaldehyde and BF3·Et2O cleanly produces E-23(23′)-benzylidenspirostanes in good yields in a reaction pathway which consists on an aldol reaction followed by a dehydration step. The obtained E-23(23′)-benzylidenspirostanes can be easily converted to dinorcholanic lactones by treatment with CrO3 in acetic acid. The synthetic sequence to dinorcholanic lactones is compatible with the presence of double bonds and carbonyl groups in the steroid framework.
Figure optionsDownload as PowerPoint slideHighlights
► Reaction of spirostanes with benzaldehyde produces E-23(23′)-benzylidenspirostanes.
► The reaction pathway consists of an aldol reaction followed by a dehydration step.
► Benzylidenspirostanes are oxidized to dinorcholanic lactones with CrO3 in acetic.
► X-ray studies of the obtained compounds confirmed the proposed structures.
Journal: Steroids - Volume 77, Issue 7, June 2012, Pages 819–828