| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 2041719 | 1073171 | 2016 | 14 صفحه PDF | دانلود رایگان |
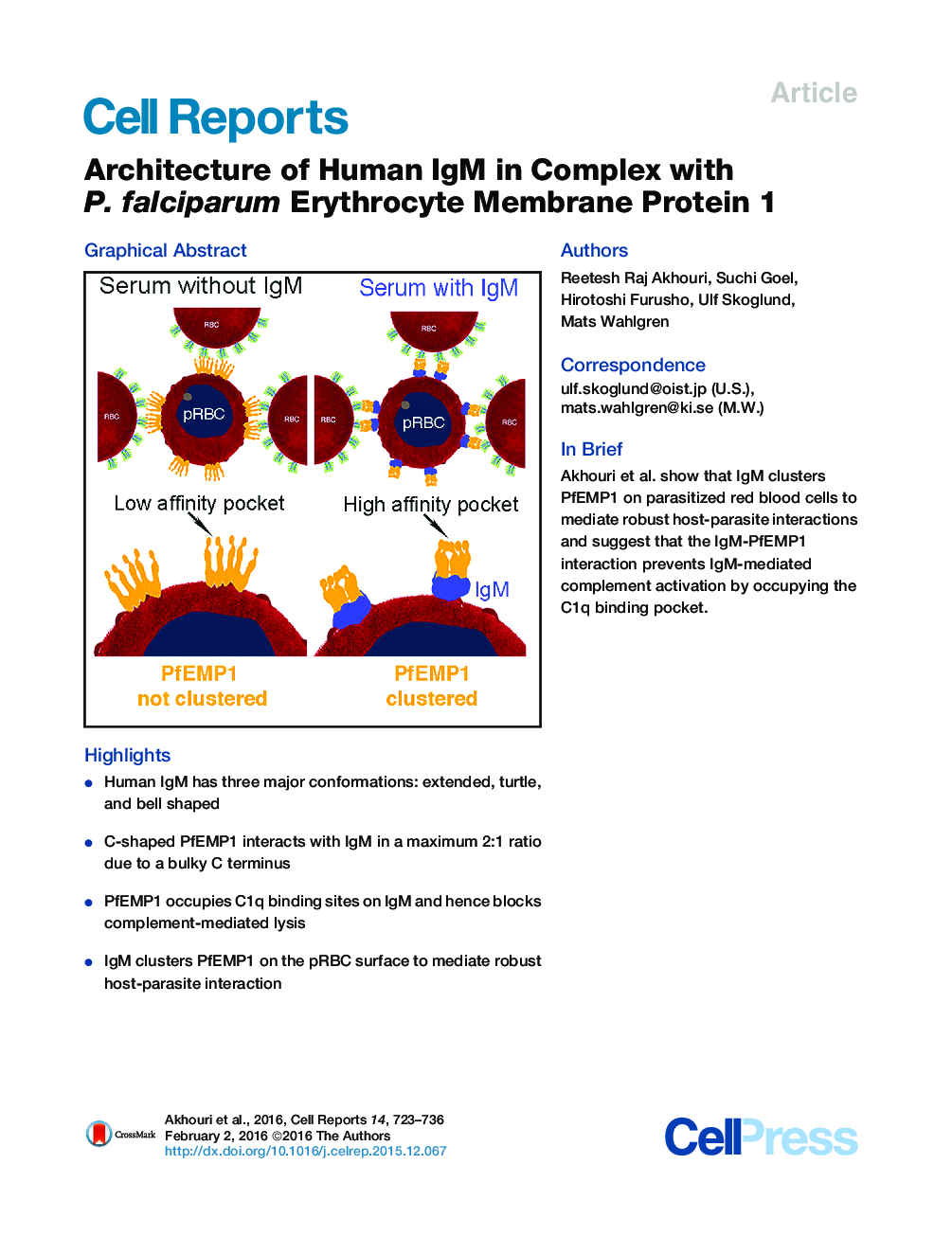
• Human IgM has three major conformations: extended, turtle, and bell shaped
• C-shaped PfEMP1 interacts with IgM in a maximum 2:1 ratio due to a bulky C terminus
• PfEMP1 occupies C1q binding sites on IgM and hence blocks complement-mediated lysis
• IgM clusters PfEMP1 on the pRBC surface to mediate robust host-parasite interaction
SummaryPlasmodium falciparum virulence is associated with sequestration of infected erythrocytes. Microvascular binding mediated by PfEMP1 in complex with non-immune immunoglobulin M (IgM) is common among parasites that cause both severe childhood malaria and pregnancy-associated malaria. Here, we present cryo-molecular electron tomography structures of human IgM, PfEMP1 and their complex. Three-dimensional reconstructions of IgM reveal that it has a dome-like core, randomly oriented Fab2s units, and the overall shape of a turtle. PfEMP1 is a C- shaped molecule with a flexible N terminus followed by an arc-shaped backbone and a bulky C terminus that interacts with IgM. Our data demonstrate that the PfEMP1 binding pockets on IgM overlap with those of C1q, and the bulkiness of PfEMP1 limits the capacity of IgM to interact with PfEMP1. We suggest that P. falciparum exploits IgM to cluster PfEMP1 into an organized matrix to augment its affinity to host cell receptors.
Graphical AbstractFigure optionsDownload as PowerPoint slide
Journal: - Volume 14, Issue 4, 2 February 2016, Pages 723–736