| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1228900 | 1495225 | 2015 | 8 صفحه PDF | دانلود رایگان |
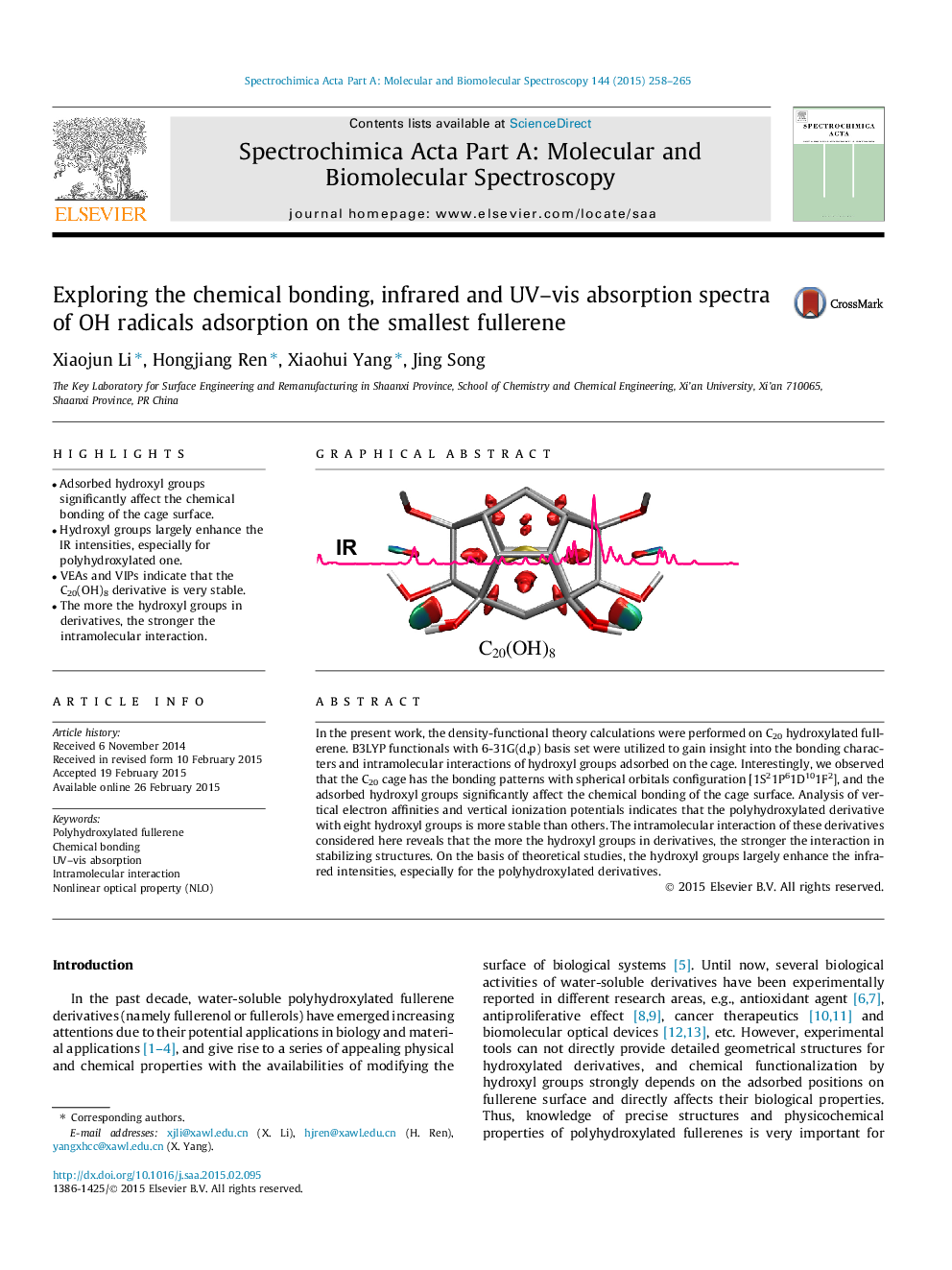
• Adsorbed hydroxyl groups significantly affect the chemical bonding of the cage surface.
• Hydroxyl groups largely enhance the IR intensities, especially for polyhydroxylated one.
• VEAs and VIPs indicate that the C20(OH)8 derivative is very stable.
• The more the hydroxyl groups in derivatives, the stronger the intramolecular interaction.
In the present work, the density-functional theory calculations were performed on C20 hydroxylated fullerene. B3LYP functionals with 6-31G(d,p) basis set were utilized to gain insight into the bonding characters and intramolecular interactions of hydroxyl groups adsorbed on the cage. Interestingly, we observed that the C20 cage has the bonding patterns with spherical orbitals configuration [1S21P61D101F2], and the adsorbed hydroxyl groups significantly affect the chemical bonding of the cage surface. Analysis of vertical electron affinities and vertical ionization potentials indicates that the polyhydroxylated derivative with eight hydroxyl groups is more stable than others. The intramolecular interaction of these derivatives considered here reveals that the more the hydroxyl groups in derivatives, the stronger the interaction in stabilizing structures. On the basis of theoretical studies, the hydroxyl groups largely enhance the infrared intensities, especially for the polyhydroxylated derivatives.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 144, 5 June 2015, Pages 258–265