| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1229631 | 1495236 | 2014 | 6 صفحه PDF | دانلود رایگان |
• PECs of the OBr2− and OCl2− dianions are calculated using QCISD(T) and CIS methods.
• Ground state of both dianions are metastable without any vibrational states.
• Metastable excited state B2Σ/A2Σ of OBr2−/OCl2− suites several vibrational bound states.
• Metastable OBr2− and OCl2− dianions can be formed via electron capture processes.
• The OBr− and OCl− singly charged anions are stable in their ground states.
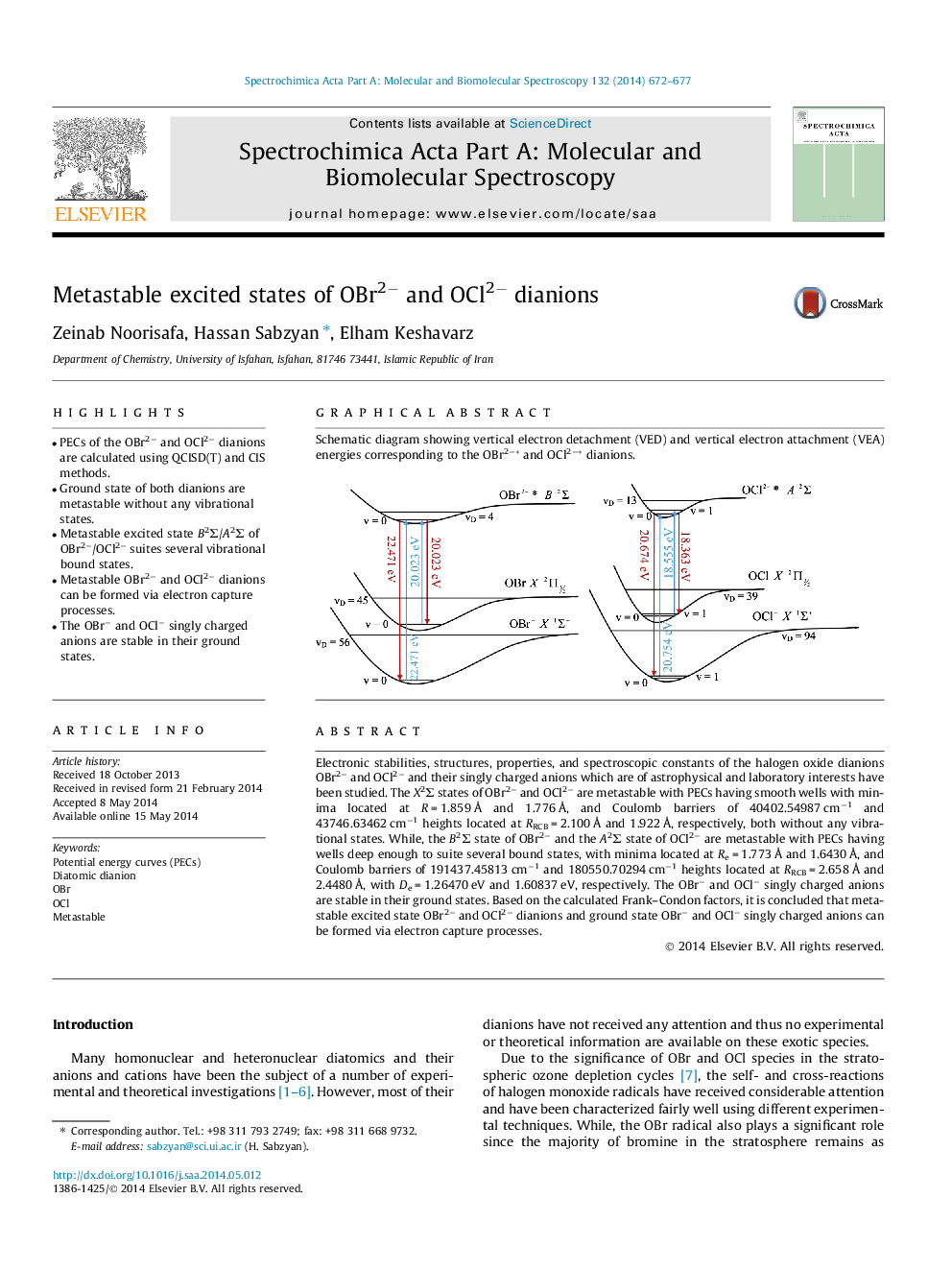
Electronic stabilities, structures, properties, and spectroscopic constants of the halogen oxide dianions OBr2− and OCl2− and their singly charged anions which are of astrophysical and laboratory interests have been studied. The X2Σ states of OBr2− and OCl2− are metastable with PECs having smooth wells with minima located at R = 1.859 Å and 1.776 Å, and Coulomb barriers of 40402.54987 cm−1 and 43746.63462 cm−1 heights located at RRCB = 2.100 Å and 1.922 Å, respectively, both without any vibrational states. While, the B2Σ state of OBr2− and the A2Σ state of OCl2− are metastable with PECs having wells deep enough to suite several bound states, with minima located at Re = 1.773 Å and 1.6430 Å, and Coulomb barriers of 191437.45813 cm−1 and 180550.70294 cm−1 heights located at RRCB = 2.658 Å and 2.4480 Å, with De = 1.26470 eV and 1.60837 eV, respectively. The OBr− and OCl− singly charged anions are stable in their ground states. Based on the calculated Frank–Condon factors, it is concluded that metastable excited state OBr2− and OCl2− dianions and ground state OBr− and OCl− singly charged anions can be formed via electron capture processes.
Schematic diagram showing vertical electron detachment (VED) and vertical electron attachment (VEA) energies corresponding to the OBr2−* and OCl2−* dianions.Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 132, 11 November 2014, Pages 672–677