| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1229646 | 1495236 | 2014 | 9 صفحه PDF | دانلود رایگان |
• The interactions between BSA and FNC or analogs have been investigated.
• Hydrophobic interactions play major role in the binding process.
• The influence of molecular structure on the binding aspects has been investigated.
• Molecular docking was also applied in the binding study.
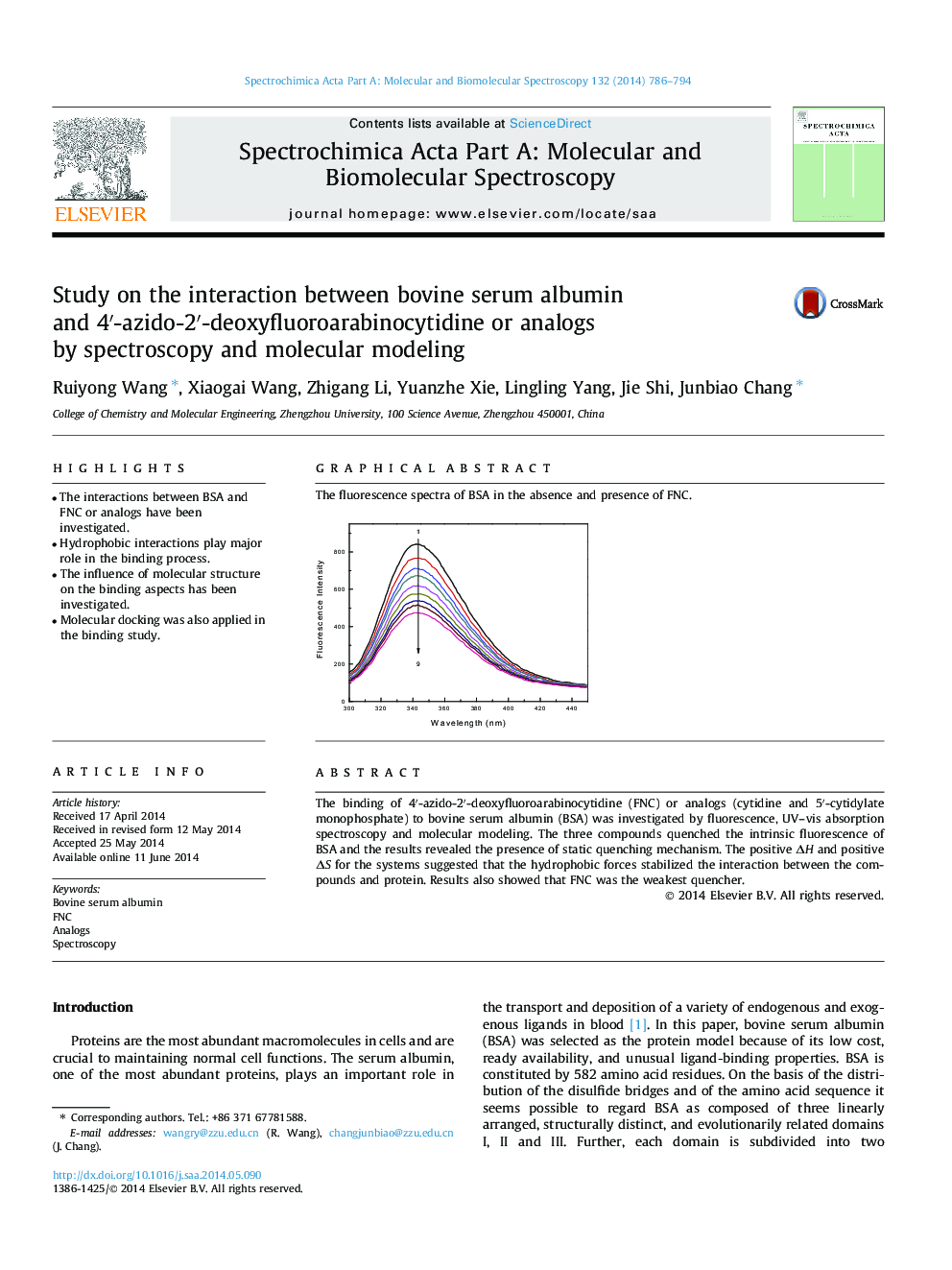
The binding of 4′-azido-2′-deoxyfluoroarabinocytidine (FNC) or analogs (cytidine and 5′-cytidylate monophosphate) to bovine serum albumin (BSA) was investigated by fluorescence, UV–vis absorption spectroscopy and molecular modeling. The three compounds quenched the intrinsic fluorescence of BSA and the results revealed the presence of static quenching mechanism. The positive ΔH and positive ΔS for the systems suggested that the hydrophobic forces stabilized the interaction between the compounds and protein. Results also showed that FNC was the weakest quencher.
The fluorescence spectra of BSA in the absence and presence of FNC.Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 132, 11 November 2014, Pages 786–794