| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1231090 | 1495273 | 2012 | 7 صفحه PDF | دانلود رایگان |
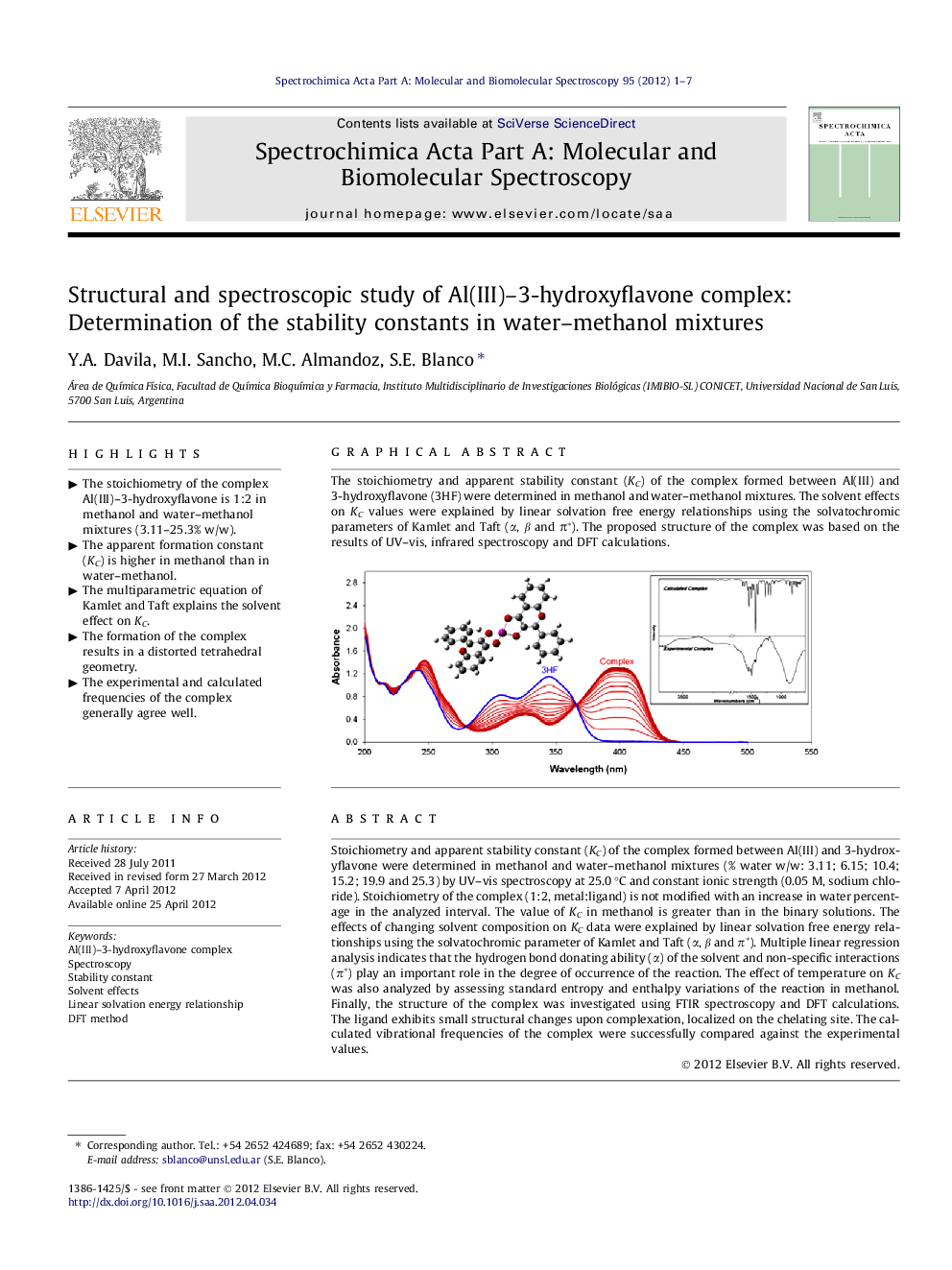
Stoichiometry and apparent stability constant (KC) of the complex formed between Al(III) and 3-hydroxyflavone were determined in methanol and water–methanol mixtures (% water w/w: 3.11; 6.15; 10.4; 15.2; 19.9 and 25.3) by UV–vis spectroscopy at 25.0 °C and constant ionic strength (0.05 M, sodium chloride). Stoichiometry of the complex (1:2, metal:ligand) is not modified with an increase in water percentage in the analyzed interval. The value of KC in methanol is greater than in the binary solutions. The effects of changing solvent composition on KC data were explained by linear solvation free energy relationships using the solvatochromic parameter of Kamlet and Taft (α, β and π*). Multiple linear regression analysis indicates that the hydrogen bond donating ability (α) of the solvent and non-specific interactions (π*) play an important role in the degree of occurrence of the reaction. The effect of temperature on KC was also analyzed by assessing standard entropy and enthalpy variations of the reaction in methanol. Finally, the structure of the complex was investigated using FTIR spectroscopy and DFT calculations. The ligand exhibits small structural changes upon complexation, localized on the chelating site. The calculated vibrational frequencies of the complex were successfully compared against the experimental values.
The stoichiometry and apparent stability constant (KC) of the complex formed between Al(III) and 3-hydroxyflavone (3HF) were determined in methanol and water–methanol mixtures. The solvent effects on KC values were explained by linear solvation free energy relationships using the solvatochromic parameters of Kamlet and Taft (α, β and π*). The proposed structure of the complex was based on the results of UV–vis, infrared spectroscopy and DFT calculations.Figure optionsDownload as PowerPoint slideHighlights
► The stoichiometry of the complex Al(III)–3-hydroxyflavone is 1:2 in methanol and water–methanol mixtures (3.11–25.3% w/w).
► The apparent formation constant (KC) is higher in methanol than in water–methanol.
► The multiparametric equation of Kamlet and Taft explains the solvent effect on KC.
► The formation of the complex results in a distorted tetrahedral geometry.
► The experimental and calculated frequencies of the complex generally agree well.
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 95, September 2012, Pages 1–7