| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1285536 | 1497927 | 2016 | 10 صفحه PDF | دانلود رایگان |
• Electrodeposited hybrid 3D hierarchical Co–MnHCF thin films surface were studied.
• Activities of Co–MnHCF for supercapacitor and water oxidation were tested.
• Improved performance of Co–MnHCF and its derived mixed oxide films were discussed.
• Both surface morphology and mixed oxide effects of Co–MnHCF were presented.
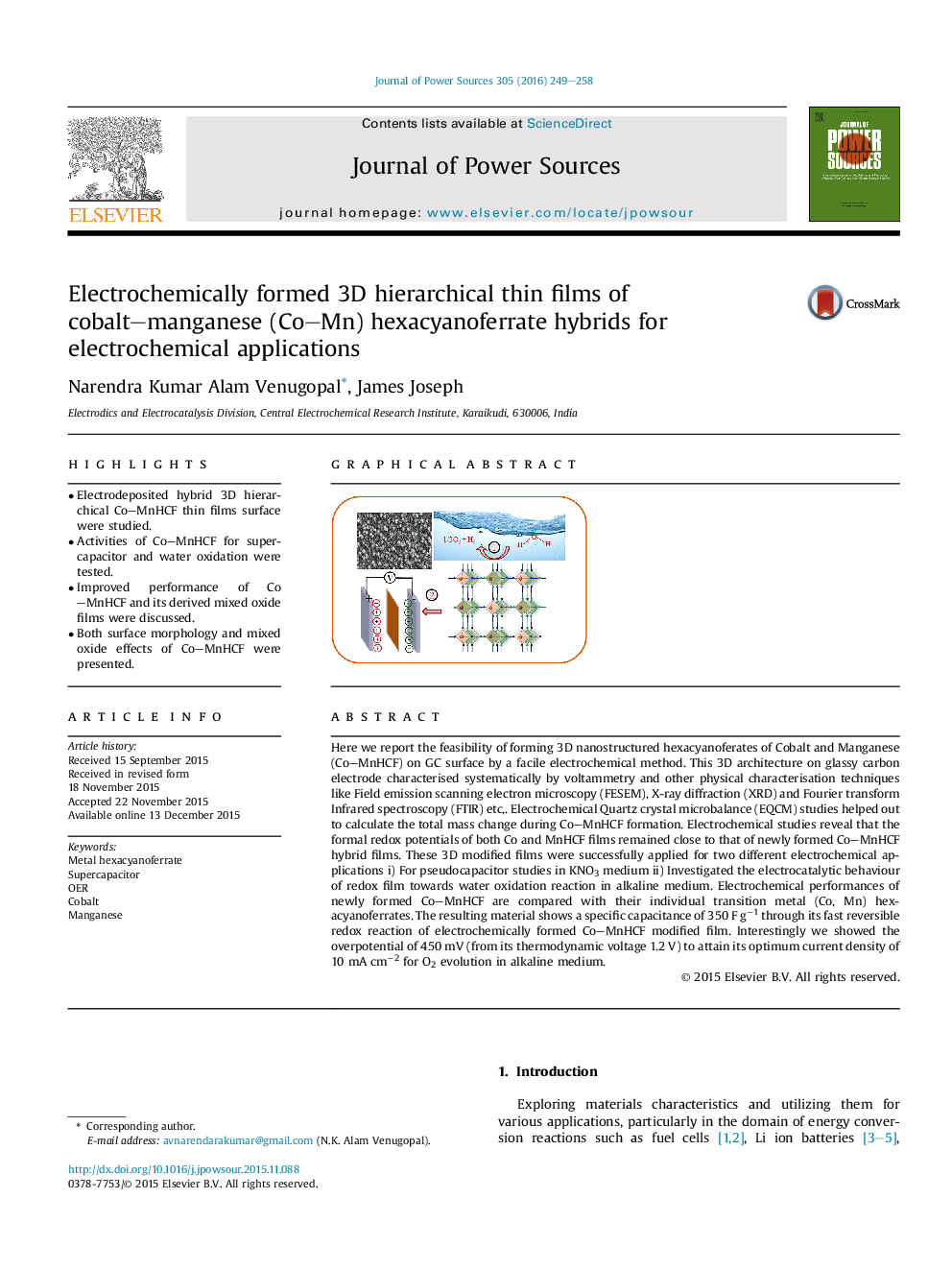
Here we report the feasibility of forming 3D nanostructured hexacyanoferates of Cobalt and Manganese (Co–MnHCF) on GC surface by a facile electrochemical method. This 3D architecture on glassy carbon electrode characterised systematically by voltammetry and other physical characterisation techniques like Field emission scanning electron microscopy (FESEM), X-ray diffraction (XRD) and Fourier transform Infrared spectroscopy (FTIR) etc,. Electrochemical Quartz crystal microbalance (EQCM) studies helped out to calculate the total mass change during Co–MnHCF formation. Electrochemical studies reveal that the formal redox potentials of both Co and MnHCF films remained close to that of newly formed Co–MnHCF hybrid films. These 3D modified films were successfully applied for two different electrochemical applications i) For pseudocapacitor studies in KNO3 medium ii) Investigated the electrocatalytic behaviour of redox film towards water oxidation reaction in alkaline medium. Electrochemical performances of newly formed Co–MnHCF are compared with their individual transition metal (Co, Mn) hexacyanoferrates. The resulting material shows a specific capacitance of 350 F g−1 through its fast reversible redox reaction of electrochemically formed Co–MnHCF modified film. Interestingly we showed the overpotential of 450 mV (from its thermodynamic voltage 1.2 V) to attain its optimum current density of 10 mA cm−2 for O2 evolution in alkaline medium.
Electrochemically deposited 3D hierarchical thin film of Co–MnHCF (hybrid metal hexacyanoferrates) reveals a unique electrocatalytic activity in terms of oxygen evolution reaction (OER) and also for supercapacitor applications. The dual benefits offered by newly formed Co–MnHCF hybrid is because of its material morphology (accessibility) and its conversion to mixed oxides.Figure optionsDownload as PowerPoint slide
Journal: Journal of Power Sources - Volume 305, 15 February 2016, Pages 249–258