| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1360400 | 981434 | 2008 | 12 صفحه PDF | دانلود رایگان |
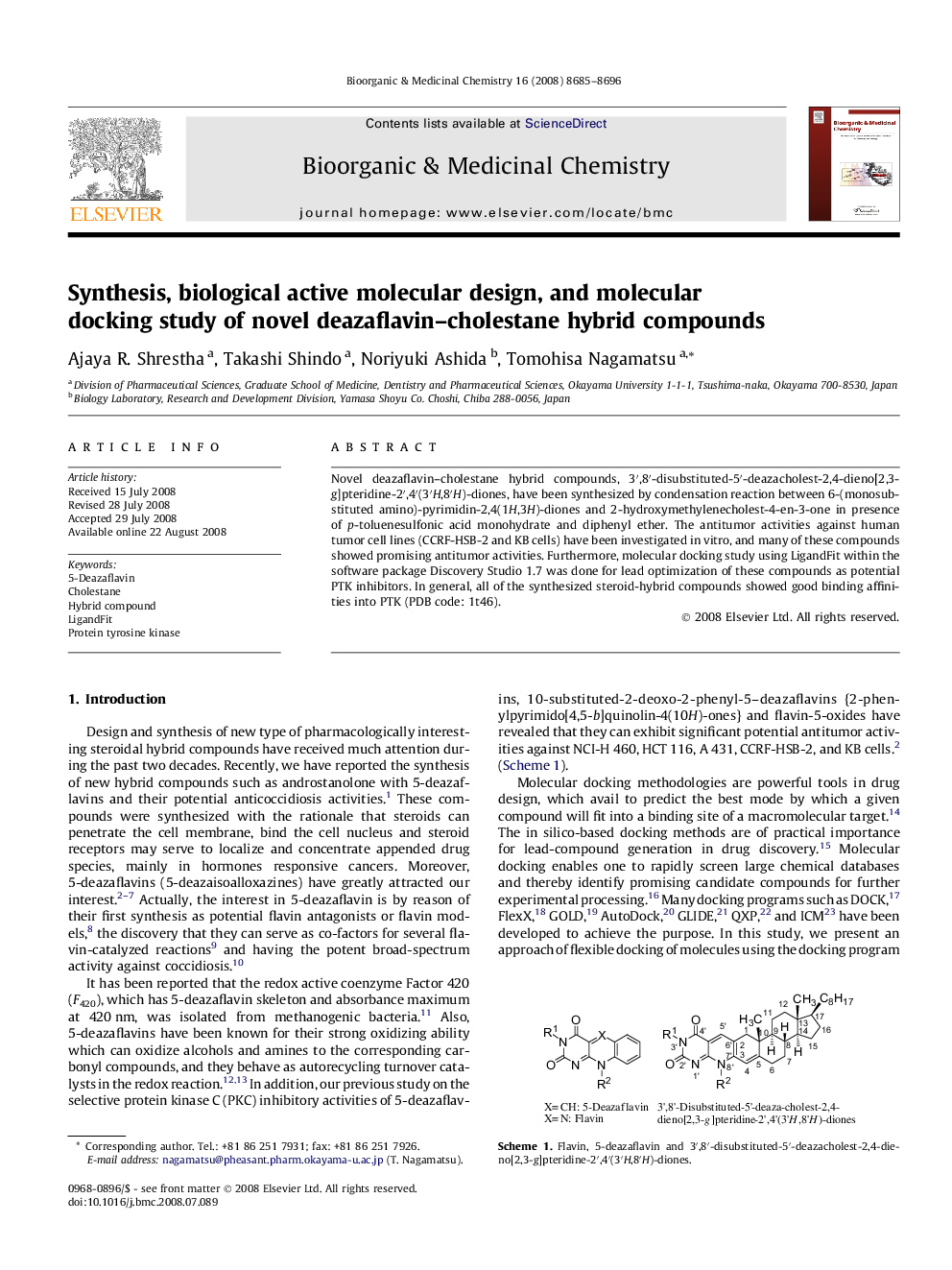
Novel deazaflavin–cholestane hybrid compounds, 3′,8′-disubstituted-5′-deazacholest-2,4-dieno[2,3-g]pteridine-2′,4′(3′H,8′H)-diones, have been synthesized by condensation reaction between 6-(monosubstituted amino)-pyrimidin-2,4(1H,3H)-diones and 2-hydroxymethylenecholest-4-en-3-one in presence of p-toluenesulfonic acid monohydrate and diphenyl ether. The antitumor activities against human tumor cell lines (CCRF-HSB-2 and KB cells) have been investigated in vitro, and many of these compounds showed promising antitumor activities. Furthermore, molecular docking study using LigandFit within the software package Discovery Studio 1.7 was done for lead optimization of these compounds as potential PTK inhibitors. In general, all of the synthesized steroid-hybrid compounds showed good binding affinities into PTK (PDB code: 1t46).
Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry - Volume 16, Issue 18, 15 September 2008, Pages 8685–8696