| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1363254 | 981507 | 2007 | 12 صفحه PDF | دانلود رایگان |
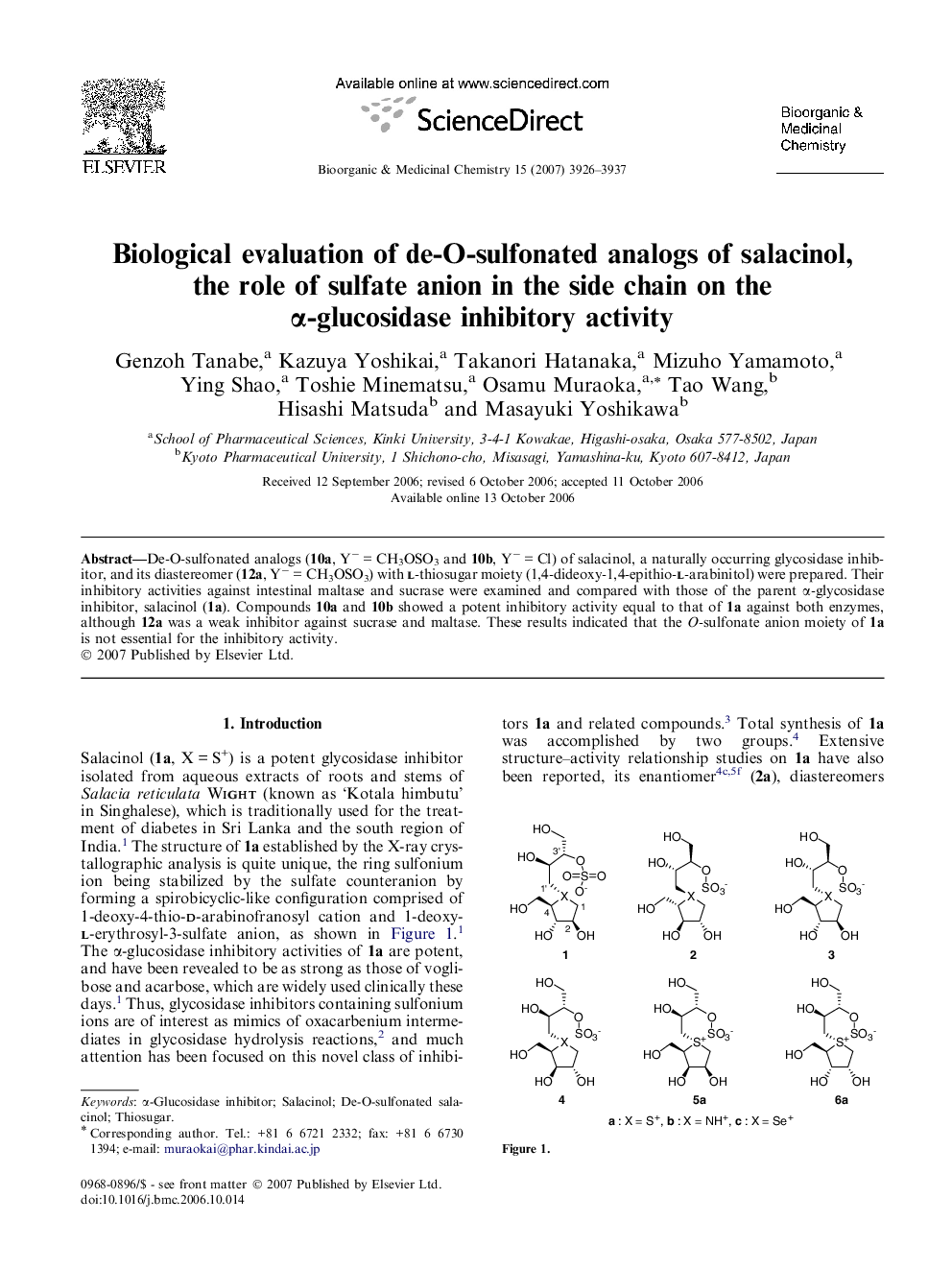
De-O-sulfonated analogs (10a, Y− = CH3OSO3 and 10b, Y− = Cl) of salacinol, a naturally occurring glycosidase inhibitor, and its diastereomer (12a, Y− = CH3OSO3) with l-thiosugar moiety (1,4-dideoxy-1,4-epithio-l-arabinitol) were prepared. Their inhibitory activities against intestinal maltase and sucrase were examined and compared with those of the parent α-glycosidase inhibitor, salacinol (1a). Compounds 10a and 10b showed a potent inhibitory activity equal to that of 1a against both enzymes, although 12a was a weak inhibitor against sucrase and maltase. These results indicated that the O-sulfonate anion moiety of 1a is not essential for the inhibitory activity.
Two de-O-sulfonated salacinols (10a and 10b) and the diastereomer (12a) were synthesized and their inhibitory activity against intestinal α-glucosidase was examined. A potent inhibitory activity of 10a and 10b equal to that of 1a against α-glucosidase indicated that the O-sulfonate anion moiety of 1a is not essential for the inhibitory activity.Figure optionsDownload as PowerPoint slide
Journal: Bioorganic & Medicinal Chemistry - Volume 15, Issue 11, 1 June 2007, Pages 3926–3937